Precatalyst-Enabled Selectivity: Enantioselective NiH-Catalyzed anti-Hydrometalative Cyclization of Alkynones to Endo- and Heterocyclic Allylic Alcohols
Xiao-Wen Zhang
Sauvage Center for Molecular Sciences, Engineering Research Center of Organosilicon Compounds & Materials (Ministry of Education), College of Chemistry and Molecular Sciences, Wuhan University, 299 Bayi Rd, Wuhan, 430072 China
Search for more papers by this authorMing-Hui Zhu
Sauvage Center for Molecular Sciences, Engineering Research Center of Organosilicon Compounds & Materials (Ministry of Education), College of Chemistry and Molecular Sciences, Wuhan University, 299 Bayi Rd, Wuhan, 430072 China
Search for more papers by this authorHai-Xiang Zeng
Sauvage Center for Molecular Sciences, Engineering Research Center of Organosilicon Compounds & Materials (Ministry of Education), College of Chemistry and Molecular Sciences, Wuhan University, 299 Bayi Rd, Wuhan, 430072 China
Search for more papers by this authorQi-Yang Li
Sauvage Center for Molecular Sciences, Engineering Research Center of Organosilicon Compounds & Materials (Ministry of Education), College of Chemistry and Molecular Sciences, Wuhan University, 299 Bayi Rd, Wuhan, 430072 China
Search for more papers by this authorCorresponding Author
Prof. Wen-Bo Liu
Sauvage Center for Molecular Sciences, Engineering Research Center of Organosilicon Compounds & Materials (Ministry of Education), College of Chemistry and Molecular Sciences, Wuhan University, 299 Bayi Rd, Wuhan, 430072 China
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Rd, Shanghai, 200032 China
Search for more papers by this authorXiao-Wen Zhang
Sauvage Center for Molecular Sciences, Engineering Research Center of Organosilicon Compounds & Materials (Ministry of Education), College of Chemistry and Molecular Sciences, Wuhan University, 299 Bayi Rd, Wuhan, 430072 China
Search for more papers by this authorMing-Hui Zhu
Sauvage Center for Molecular Sciences, Engineering Research Center of Organosilicon Compounds & Materials (Ministry of Education), College of Chemistry and Molecular Sciences, Wuhan University, 299 Bayi Rd, Wuhan, 430072 China
Search for more papers by this authorHai-Xiang Zeng
Sauvage Center for Molecular Sciences, Engineering Research Center of Organosilicon Compounds & Materials (Ministry of Education), College of Chemistry and Molecular Sciences, Wuhan University, 299 Bayi Rd, Wuhan, 430072 China
Search for more papers by this authorQi-Yang Li
Sauvage Center for Molecular Sciences, Engineering Research Center of Organosilicon Compounds & Materials (Ministry of Education), College of Chemistry and Molecular Sciences, Wuhan University, 299 Bayi Rd, Wuhan, 430072 China
Search for more papers by this authorCorresponding Author
Prof. Wen-Bo Liu
Sauvage Center for Molecular Sciences, Engineering Research Center of Organosilicon Compounds & Materials (Ministry of Education), College of Chemistry and Molecular Sciences, Wuhan University, 299 Bayi Rd, Wuhan, 430072 China
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Rd, Shanghai, 200032 China
Search for more papers by this authorGraphical Abstract
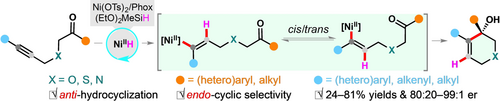
An unprecedented NiH-catalyzed enantio-, regio-, and anti-selective intramolecular coupling of alkynones to construct O-, N-, S-containing endocyclic allylic alcohols was developed. The choice of metal precursors plays a key role in tuning the regio- and enantio-selectivity. This study offers a new anti-hydrocyclization mode for enantioselective hydrofunctionalization of alkynes.
Abstract
A highly enantioselective NiH-catalyzed hydrocyclization of alkynones with unparalleled anti- and endocyclic selectivities is described. The choice of the precatalysts has significant influence in tuning the regio- and enantioselectivity. Using Ni(OTs)2/Phox as a precatalyst and (EtO)2MeSiH as a hydride source, an array of enantioenriched O-, N-, and S-containing heterocyclic tertiary allylic alcohols are obtained in 24–81 % yields with 80:20–99:1 er. Mechanistic investigations and synthetic application are also carried out. This study represents an efficient access to a set of allylic alcohols that are unable to access by the state-of-the-art coupling reactions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202110815-sup-0001-cif.zip433 KB | Supporting Information |
| anie202110815-sup-0001-misc_information.pdf13.9 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Selected reviews:
- 1aF. Alonso, I. P. Beletskaya, M. Yus, Chem. Rev. 2004, 104, 3079;
- 1bR. Severin, S. Doye, Chem. Soc. Rev. 2007, 36, 1407;
- 1cX. Zeng, Chem. Rev. 2013, 113, 6864;
- 1dY. Yamamoto, Chem. Soc. Rev. 2014, 43, 1575;
- 1eA. M. Suess, G. Lalic, Synlett 2016, 27, 1165;
- 1fJ. Corpas, P. Mauleón, R. G. Arrayás, J. C. Carretero, ACS Catal. 2021, 11, 7513.
- 2Selected reviews:
- 2aY. Zheng, W. Zi, Tetrahedron Lett. 2018, 59, 2205;
- 2bM. Holmes, L. A. Schwartz, M. J. Krische, Chem. Rev. 2018, 118, 6026;
- 2cC. C. Meyer, E. Ortiz, M. J. Krische, Chem. Rev. 2020, 120, 3721.
- 3Selected examples:
- 3aJ.-R. Kong, M.-Y. Ngai, M. J. Krische, J. Am. Chem. Soc. 2006, 128, 718;
- 3bK. Masuda, N. Sakiyama, R. Tanaka, K. Noguchi, K. Tanaka, J. Am. Chem. Soc. 2011, 133, 6918;
- 3cC. C. Bausch, R. L. Patman, B. Breit, M. J. Krische, Angew. Chem. Int. Ed. 2011, 50, 5687; Angew. Chem. 2011, 123, 5805;
- 3dM.-Y. Ngai, A. Barchuk, M. J. Krische, J. Am. Chem. Soc. 2007, 129, 280;
- 3eP. Gandeepan, C.-H. Cheng, Acc. Chem. Res. 2015, 48, 1194;
- 3fY.-L. Li, S.-Q. Zhang, J. Chen, J.-B. Xia, J. Am. Chem. Soc. 2021, 143, 7306;
- 3gJ. Montgomery, Angew. Chem. Int. Ed. 2004, 43, 3890; Angew. Chem. 2004, 116, 3980;
- 3hR. M. Moslin, K. Miller-Moslin, T. F. Jamison, Chem. Commun. 2007, 4441;
- 3iM. R. Chaulagain, G. J. Sormunen, J. Montgomery, J. Am. Chem. Soc. 2007, 129, 9568;
- 3jA. B. Bahadoor, A. Flyer, G. C. Micalizio, J. Am. Chem. Soc. 2005, 127, 3694;
- 3kK. Shen, X. Han, X. Lu, Org. Lett. 2013, 15, 1732;
- 3lX.-T. Liu, X.-Y. Han, Y. Wu, Y.-Y. Sun, L. Gao, Z. Huang, Q.-W. Zhang, J. Am. Chem. Soc. 2021, 143, 11309.
- 4Selected examples:
- 4aB. M. Trost, Z. T. Ball, J. Am. Chem. Soc. 2005, 127, 17644;
- 4bB. Sundararaju, A. Fürstner, Angew. Chem. Int. Ed. 2013, 52, 14050; Angew. Chem. 2013, 125, 14300;
- 4cC. Gunanathan, M. Hölscher, F. Pan, W. Leitner, J. Am. Chem. Soc. 2012, 134, 14349;
- 4dM. Hari Babu, G. Ranjith Kumar, R. Kant, M. Sridhar Reddy, Chem. Commun. 2017, 53, 3894;
- 4eA. Yamamoto, M. Suginome, J. Am. Chem. Soc. 2005, 127, 15706;
- 4fX. Wang, Y. Liu, R. Martin, J. Am. Chem. Soc. 2015, 137, 6476;
- 4gK. Tanaka, G. C. Fu, J. Am. Chem. Soc. 2001, 123, 11492;
- 4hK. Tanaka, G. C. Fu, Angew. Chem. Int. Ed. 2002, 41, 1607;
10.1002/1521-3773(20020503)41:9<1607::AID-ANIE1607>3.0.CO;2-Q CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 1677;
- 4iFor a review: S. E. Bottcher, L. E. Hutchinson, D. J. Wilger, Synthesis 2020, 52, 2807.
- 5
- 5aE. Oblinger, J. Montgomery, J. Am. Chem. Soc. 1997, 119, 9065;
- 5bE. P. Jackson, H. A. Malik, G. J. Sormunen, R. D. Baxter, P. Liu, H. Wang, A.-R. Shareef, J. Montgomery, Acc. Chem. Res. 2015, 48, 1736.
- 6
- 6aW.-S. Huang, J. Chan, T. F. Jamison, Org. Lett. 2000, 2, 4221;
- 6bK. M. Miller, W.-S. Huang, T. F. Jamison, J. Am. Chem. Soc. 2003, 125, 3442;
- 6cE. A. Standley, S. Z. Tasker, K. L. Jensen, T. F. Jamison, Acc. Chem. Res. 2015, 48, 1503.
- 7
- 7aY. Sato, M. Takimoto, K. Hayashi, T. Katsuhara, K. Takagi, M. Mori, J. Am. Chem. Soc. 1994, 116, 9771;
- 7bS. Ikeda, Angew. Chem. Int. Ed. 2003, 42, 5120; Angew. Chem. 2003, 115, 5276;
- 7cY. Yang, S.-F. Zhu, H.-F. Duan, C.-Y. Zhou, L.-X. Wang, Q.-L. Zhou, J. Am. Chem. Soc. 2007, 129, 2248;
- 7dS. Ogoshi, T. Arai, M. Ohashi, H. Kurosawa, Chem. Commun. 2008, 1347.
- 8H. A. Malik, R. D. Baxter, J. Montgomery in Catalysis without Precious Metals, 1st ed. (Ed.: R. M. Bullock), Wiley-VCH, Weinheim, 2010, pp. 181.
10.1002/9783527631582.ch8 Google Scholar
- 9
- 9aE. A. Colby, K. C. O'Brien, T. F. Jamison, J. Am. Chem. Soc. 2004, 126, 998;
- 9bB. Knapp-Reed, G. M. Mahandru, J. Montgomery, J. Am. Chem. Soc. 2005, 127, 13156;
- 9cC. C. Chrovian, B. Knapp-Reed, J. Montgomery, Org. Lett. 2008, 10, 811;
- 9dA.-R. Shareef, D. H. Sherman, J. Montgomery, Chem. Sci. 2012, 3, 892;
- 9eS. Kitahata, A. Katsuyama, S. Ichikawa, Org. Lett. 2020, 22, 2697.
- 10
- 10aW. Fu, M. Nie, A. Wang, Z. Cao, W. Tang, Angew. Chem. Int. Ed. 2015, 54, 2520; Angew. Chem. 2015, 127, 2550;
- 10bG. Liu, W. Fu, X. Mu, T. Wu, M. Nie, K. Li, X. Xu, W. Tang, Commun. Chem. 2018, 1, 90.
- 11
- 11aY. Mu, T. Zhang, Y. Cheng, W. Fu, Z. Wei, W. Chen, G. Liu, Catal. Sci. Technol. 2021, 11, 2306;
- 11bW. Chen, Y. Cheng, T. Zhang, Y. Mu, W. Jia, G. Liu, J. Org. Chem. 2021, 86, 5166.
- 12
- 12aL. She, X. Li, H. Sun, J. Ding, M. Frey, H.-F. Klein, Organometallics 2007, 26, 566;
- 12bG. L. O. Wilson, M. Abraha, J. A. Krause, H. Guan, Dalton Trans. 2015, 44, 12128.
- 13J. M. Huggins, R. G. Bergman, J. Am. Chem. Soc. 1979, 101, 4410.
- 14X. Zhang, X. Xie, Y. Liu, Chem. Sci. 2016, 7, 5815.
- 15
- 15aC. Clarke, C. A. Incerti-Pradillos, H. W. Lam, J. Am. Chem. Soc. 2016, 138, 8068;
- 15bC. Yap, G. M. J. Lenagh-Snow, S. N. Karad, W. Lewis, L. J. Diorazio, H. W. Lam, Angew. Chem. Int. Ed. 2017, 56, 8216; Angew. Chem. 2017, 129, 8328;
- 15cS. N. Karad, H. Panchal, C. Clarke, W. Lewis, H. W. Lam, Angew. Chem. Int. Ed. 2018, 57, 9122; Angew. Chem. 2018, 130, 9260;
- 15dH. Green, S. P. Argent, H. W. Lam, Chem. Eur. J. 2021, 27, 5897;
- 15eFor a recent example, J. Tian, W. Li, R. Li, L. He, H. Lv, Chin. Chem. Lett. 2021, https://doi.org/10.1016/j.cclet.2021.06.006.
- 16Z. Lu, X.-D. Hu, H. Zhang, X.-W. Zhang, J. Cai, M. Usman, H. Cong, W.-B. Liu, J. Am. Chem. Soc. 2020, 142, 7328.
- 17G. Helmchen, A. Pfaltz, Acc. Chem. Res. 2000, 33, 336.
- 18C. F. Lochow, R. G. Miller, J. Org. Chem. 1976, 41, 3020.
- 19
- 19aG. C. Lloyd-Jones, C. P. Butts, Tetrahedron 1998, 54, 901;
- 19bK. L. Bray, C. P. Butts, G. C. Lloyd-Jones, M. Murray, J. Chem. Soc. Dalton Trans. 1998, 1421.
- 20T. Satyanarayana, S. Abraham, H. B. Kagan, Angew. Chem. Int. Ed. 2009, 48, 456; Angew. Chem. 2009, 121, 464.
- 21The possibility of a homochiral complex Ni(L1)2, formed by self-recognition of L1, as the catalytic species cannot be ruled out by the linear effect experiment.
- 22
- 22aK. W. Shimkin, J. Montgomery, J. Am. Chem. Soc. 2018, 140, 7074;
- 22bZ. Zhou, W. Liu, W. Kong, Org. Lett. 2020, 22, 6982;
- 22cY. He, Y. Cai, S. Zhu, J. Am. Chem. Soc. 2017, 139, 1061;
- 22dY. Zhang, B. Han, S. Zhu, Angew. Chem. Int. Ed. 2019, 58, 13860; Angew. Chem. 2019, 131, 13998;
- 22eJ. Diccianni, Q. Lin, T. Diao, Acc. Chem. Res. 2020, 53, 906;
- 22fY. Chen, Z. Ding, Y. Wang, W. Liu, W. Kong, Angew. Chem. Int. Ed. 2021, 60, 5273; Angew. Chem. 2021, 133, 5333.
- 23For mechanisms regard to the formation of the minor products, see Scheme S8 in the Supporting Information.
- 24R. Guo, X. Zheng, D. Zhang, G. Zhang, Chem. Sci. 2017, 8, 3002.
- 25F. Serpier, J.-L. Brayer, B. Folléas, S. Darses, Org. Lett. 2015, 17, 5496.