3,4,5-Trimethoxy Substitution on an N-DMBI Dopant with New N-Type Polymers: Polymer-Dopant Matching for Improved Conductivity-Seebeck Coefficient Relationship
Jinfeng Han
Department of Materials Science and Engineering, Johns Hopkins University, 3400 North Charles Street, Baltimore, Maryland, 21218 USA
Search for more papers by this authorArlene Chiu
Department of Electrical and Computer Engineering, Johns Hopkins University, 3400 North Charles Street, Baltimore, Maryland, 21218 USA
Search for more papers by this authorConnor Ganley
Department of Chemical and Biomolecular Engineering, Johns Hopkins University, Baltimore, Maryland, 21218 USA
Search for more papers by this authorProf. Patty McGuiggan
Department of Materials Science and Engineering, Johns Hopkins University, 3400 North Charles Street, Baltimore, Maryland, 21218 USA
Search for more papers by this authorProf. Susanna M. Thon
Department of Electrical and Computer Engineering, Johns Hopkins University, 3400 North Charles Street, Baltimore, Maryland, 21218 USA
Search for more papers by this authorProf. Paulette Clancy
Department of Chemical and Biomolecular Engineering, Johns Hopkins University, Baltimore, Maryland, 21218 USA
Search for more papers by this authorCorresponding Author
Prof. Howard E. Katz
Department of Materials Science and Engineering, Johns Hopkins University, 3400 North Charles Street, Baltimore, Maryland, 21218 USA
Search for more papers by this authorJinfeng Han
Department of Materials Science and Engineering, Johns Hopkins University, 3400 North Charles Street, Baltimore, Maryland, 21218 USA
Search for more papers by this authorArlene Chiu
Department of Electrical and Computer Engineering, Johns Hopkins University, 3400 North Charles Street, Baltimore, Maryland, 21218 USA
Search for more papers by this authorConnor Ganley
Department of Chemical and Biomolecular Engineering, Johns Hopkins University, Baltimore, Maryland, 21218 USA
Search for more papers by this authorProf. Patty McGuiggan
Department of Materials Science and Engineering, Johns Hopkins University, 3400 North Charles Street, Baltimore, Maryland, 21218 USA
Search for more papers by this authorProf. Susanna M. Thon
Department of Electrical and Computer Engineering, Johns Hopkins University, 3400 North Charles Street, Baltimore, Maryland, 21218 USA
Search for more papers by this authorProf. Paulette Clancy
Department of Chemical and Biomolecular Engineering, Johns Hopkins University, Baltimore, Maryland, 21218 USA
Search for more papers by this authorCorresponding Author
Prof. Howard E. Katz
Department of Materials Science and Engineering, Johns Hopkins University, 3400 North Charles Street, Baltimore, Maryland, 21218 USA
Search for more papers by this authorGraphical Abstract
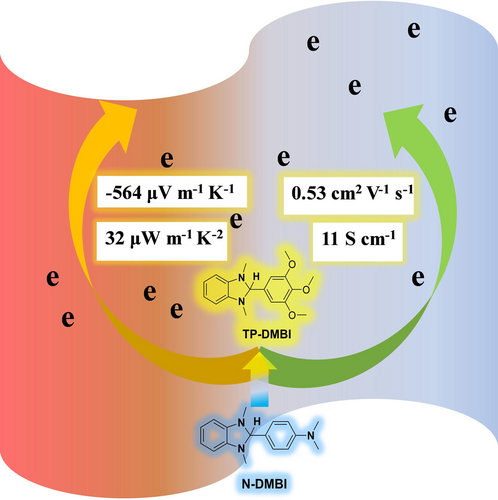
Two n-type conjugated polymers with different backbones and a n-type dopant 1,3-dimethyl-2-(3,4,5-trimethoxyphenyl)-2,3-dihydro-1H-benzo[d]imidazole (TP-DMBI) are synthesized, and electron mobility of 0.53 cm2 V−1 S−1, electrical conductivity of 11 S cm−1 and power factor 32 μW m−1 K−2 for n-type organic thermoelectrics are achieved by TP-DMBI doped films, which out-perform N-DMBI doped films.
Abstract
Achieving high electrical conductivity and thermoelectric power factor simultaneously for n-type organic thermoelectrics is still challenging. By constructing two new acceptor-acceptor n-type conjugated polymers with different backbones and introducing the 3,4,5-trimethoxyphenyl group to form the new n-type dopant 1,3-dimethyl-2-(3,4,5-trimethoxyphenyl)-2,3-dihydro-1H-benzo[d]imidazole (TP-DMBI), high electrical conductivity of 11 S cm−1 and power factor of 32 μW m−1 K−2 are achieved. Calculations using Density Functional Theory show that TP-DMBI presents a higher singly occupied molecular orbital (SOMO) energy level of −1.94 eV than that of the common dopant 4-(1, 3-dimethyl-2, 3-dihydro-1H-benzoimidazol-2-yl) phenyl) dimethylamine (N-DMBI) (−2.36 eV), which can result in a larger offset between the SOMO of dopant and lowest unoccupied molecular orbital (LUMO) of n-type polymers, though that effect may not be dominant in the present work. The doped polymer films exhibit higher Seebeck coefficient and power factor than films using N-DMBI at the same doping levels or similar electrical conductivity levels. Moreover, TP-DMBI doped polymer films offer much higher electron mobility of up to 0.53 cm2 V−1 s−1 than films with N-DMBI doping, demonstrating the potential of TP-DMBI, and 3,4,5-trialkoxy DMBIs more broadly, for high performance n-type organic thermoelectrics.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202110505-sup-0001-misc_information.pdf1.7 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. R. Murphy, J. M. J. Fréchet, Chem. Rev. 2007, 107, 1066;
- 1bJ. Li, K. Pu, Chem. Soc. Rev. 2019, 48, 38;
- 1cT. K. Das, S. Prusty, PPTEn 2012, 51, 1487.
- 2B. Lüssem, C.-M. Keum, D. Kasemann, B. Naab, Z. Bao, K. Leo, Chem. Rev. 2016, 116, 13714.
- 3B. Russ, A. Glaudell, J. J. Urban, M. L. Chabinyc, R. A. Segalman, Nat. Rev. Mater. 2016, 1, 16050.
- 4H. Li, J. Song, J. Xiao, L. Wu, H. E. Katz, L. Chen, Adv. Funct. Mater. 2020, 30, 2004378.
- 5H. Li, M. E. DeCoster, R. M. Ireland, J. Song, P. E. Hopkins, H. E. Katz, J. Am. Chem. Soc. 2017, 139, 11149.
- 6Y. Lu, Z.-D. Yu, H.-I. Un, Z.-F. Yao, H.-Y. You, W. Jin, L. Li, Z.-Y. Wang, B.-W. Dong, S. Barlow, E. Longhi, C.-a. Di, D. Zhu, J.-Y. Wang, C. Silva, S. R. Marder, J. Pei, Adv. Mater. 2021, 33, 2005946.
- 7H. Li, M. E. DeCoster, C. Ming, M. Wang, Y. Chen, P. E. Hopkins, L. Chen, H. E. Katz, Macromolecules 2019, 52, 9804.
- 8J. Ding, Z. Liu, W. Zhao, W. Jin, L. Xiang, Z. Wang, Y. Zeng, Y. Zou, F. Zhang, Y. Yi, Y. Diao, C. R. McNeill, C.-a. Di, D. Zhang, D. Zhu, Angew. Chem. Int. Ed. 2019, 58, 18994; Angew. Chem. 2019, 131, 19170.
- 9T. Park, C. Park, B. Kim, H. Shin, E. Kim, Energy Environ. Sci. 2013, 6, 788.
- 10
- 10aJ. Liu, G. Ye, B. v. d. ee, J. Dong, X. Qiu, Y. Liu, G. Portale, R. C. Chiechi, L. J. A. Koster, Adv. Mater. 2018, 30, 1804290;
- 10bJ. Liu, Y. Shi, J. Dong, M. I. Nugraha, X. Qiu, M. Su, R. C. Chiechi, D. Baran, G. Portale, X. Guo, L. J. A. Koster, ACS Energy Lett. 2019, 4, 1556;
- 10cC.-Y. Yang, M.-A. Stoeckel, T.-P. Ruoko, H.-Y. Wu, X. Liu, N. B. Kolhe, Z. Wu, Y. Puttisong, C. Musumeci, M. Massetti, H. Sun, K. Xu, D. Tu, W. M. Chen, H. Y. Woo, M. Fahlman, S. A. Jenekhe, M. Berggren, S. Fabiano, Nat. Commun. 2021, 12, 2354;
- 10dC. Dong, S. Deng, B. Meng, J. Liu, L. Wang, Angew. Chem. Int. Ed. 2021, 60, 16184; Angew. Chem. 2021, 133, 16320.
- 11
- 11aO. Bardagot, P. Kubik, T. Marszalek, P. Veyre, A. A. Medjahed, M. Sandroni, B. Grévin, S. Pouget, T. Nunes Domschke, A. Carella, S. Gambarelli, W. Pisula, R. Demadrille, Adv. Funct. Mater. 2020, 30, 2000449;
- 11bX. Zhao, D. Madan, Y. Cheng, J. Zhou, H. Li, S. M. Thon, A. E. Bragg, M. E. DeCoster, P. E. Hopkins, H. E. Katz, Adv. Mater. 2017, 29, 1606921;
- 11cJ. Han, C. Ganley, Q. Hu, X. Zhao, P. Clancy, T. P. Russell, H. E. Katz, Adv. Funct. Mater. 2021, 31, 2010567.
- 12K. Feng, H. Guo, J. Wang, Y. Shi, Z. Wu, M. Su, X. Zhang, J. H. Son, H. Y. Woo, X. Guo, J. Am. Chem. Soc. 2021, 143, 1539.
- 13X. Yan, M. Xiong, J. T. Li, S. Zhang, Z. Ahmad, Y. Lu, Z. Y. Wang, Z. F. Yao, J. Y. Wang, X. Gu, T. Lei, J. Am. Chem. Soc. 2019, 141, 20215.
- 14
- 14aY. Lu, Z. D. Yu, R. Z. Zhang, Z. F. Yao, H. Y. You, L. Jiang, H. I. Un, B. W. Dong, M. Xiong, J. Y. Wang, J. Pei, Angew. Chem. Int. Ed. 2019, 58, 11390; Angew. Chem. 2019, 131, 11512;
- 14bH. Chen, M. Moser, S. Wang, C. Jellett, K. Thorley, G. T. Harrison, X. Jiao, M. Xiao, B. Purushothaman, M. Alsufyani, H. Bristow, S. De Wolf, N. Gasparini, A. Wadsworth, C. R. McNeill, H. Sirringhaus, S. Fabiano, I. McCulloch, J. Am. Chem. Soc. 2021, 143, 260.
- 15Y. Wang, M. Nakano, T. Michinobu, Y. Kiyota, T. Mori, K. Takimiya, Macromolecules 2017, 50, 857.
- 16
- 16aJ. Han, H. Fan, Q. Zhang, Q. Hu, T. P. Russell, H. E. Katz, Adv. Funct. Mater. 2021, 31, 2005901;
- 16bC.-Y. Yang, W.-L. Jin, J. Wang, Y.-F. Ding, S. Nong, K. Shi, Y. Lu, Y.-Z. Dai, F.-D. Zhuang, T. Lei, C.-A. Di, D. Zhu, J.-Y. Wang, J. Pei, Adv. Mater. 2018, 30, 1802850.
- 17Y. Wang, K. Takimiya, Adv. Mater. 2020, 32, 2002060.
- 18
- 18aH.-I. Un, S. A. Gregory, S. K. Mohapatra, M. Xiong, E. Longhi, Y. Lu, S. Rigin, S. Jhulki, C.-Y. Yang, T. V. Timofeeva, J.-Y. Wang, S. K. Yee, S. Barlow, S. R. Marder, J. Pei, Adv. Energy Mater. 2019, 9, 1900817;
- 18bD. Yuan, D. Huang, C. Zhang, Y. Zou, C.-a. Di, X. Zhu, D. Zhu, ACS Appl. Mater. Interfaces 2017, 9, 28795.
- 19Y. Lu, Z.-D. Yu, Y. Liu, Y.-F. Ding, C.-Y. Yang, Z.-F. Yao, Z.-Y. Wang, H.-Y. You, X.-F. Cheng, B. Tang, J.-Y. Wang, J. Pei, J. Am. Chem. Soc. 2020, 142, 15340.
- 20X. Yin, F. Zhong, Z. Chen, C. Gao, G. Xie, L. Wang, C. Yang, Chem. Eng. J. 2020, 382, 122817.
- 21B. D. Naab, S. Guo, S. Olthof, E. G. B. Evans, P. Wei, G. L. Millhauser, A. Kahn, S. Barlow, S. R. Marder, Z. Bao, J. Am. Chem. Soc. 2013, 135, 15018.
- 22X.-Q. Zhu, M.-T. Zhang, A. Yu, C.-H. Wang, J.-P. Cheng, J. Am. Chem. Soc. 2008, 130, 2501.
- 23J. Han, D. Yang, Y. Wang, D. Ma, W. Qiao, Z. Y. Wang, J. Mater. Chem. C 2018, 6, 10838.
- 24B. M. Reinhard, G. Niedner-Schatteburg, J. Chem. Phys. 2003, 118, 3571.
- 25
- 25aY. Zeng, W. Zheng, Y. Guo, G. Han, Y. Yi, J. Mater. Chem. A 2020, 8, 8323;
- 25bL. Qiu, J. Liu, R. Alessandri, X. Qiu, M. Koopmans, R. W. A. Havenith, S. J. Marrink, R. C. Chiechi, L. J. A. Koster, J. C. Hummelen, J. Mater. Chem. A 2017, 5, 21234.
- 26P. Wei, J. H. Oh, G. Dong, Z. Bao, J. Am. Chem. Soc. 2010, 132, 8852.
- 27B. D. Naab, X. Gu, T. Kurosawa, J. W. F. To, A. Salleo, Z. Bao, Adv. Electron. Mater. 2016, 2, 1600004.
- 28J. L. Bredas, G. B. Street, Acc. Chem. Res. 1985, 18, 309.
- 29C. Deibel, T. Strobel, V. Dyakonov, Adv. Mater. 2010, 22, 4097.
- 30K. Shi, F. Zhang, C. A. Di, T. W. Yan, Y. Zou, X. Zhou, D. Zhu, J. Y. Wang, J. Pei, J. Am. Chem. Soc. 2015, 137, 6979.
- 31S.-G. Chen, H. M. Branz, S. S. Eaton, P. C. Taylor, R. A. Cormier, B. A. Gregg, J. Phys. Chem. B 2004, 108, 17329.
- 32Y. Lu, J.-Y. Wang, J. Pei, Chem. Mater. 2019, 31, 6412.
- 33C. J. Boyle, M. Upadhyaya, P. Wang, L. A. Renna, M. Lu-Díaz, S. Pyo Jeong, N. Hight-Huf, L. Korugic-Karasz, M. D. Barnes, Z. Aksamija, D. Venkataraman, Nat. Commun. 2019, 10, 2827.
- 34K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva, A. A. Firsov, Science 2004, 306, 666.
- 35K. Guo, J. Bai, Y. Jiang, Z. Wang, Y. Sui, Y. Deng, Y. Han, H. Tian, Y. Geng, Adv. Funct. Mater. 2018, 28, 1801097.
- 36C.-Y. Yang, Y.-F. Ding, D. Huang, J. Wang, Z.-F. Yao, C.-X. Huang, Y. Lu, H.-I. Un, F.-D. Zhuang, J.-H. Dou, C.-a. Di, D. Zhu, J.-Y. Wang, T. Lei, J. Pei, Nat. Commun. 2020, 11, 3292.