Divergent Synthesis of Skeletally Distinct Arboridinine and Arborisidine
Cheng Wang
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry & Chemical Engineering, South China University of Technology, Wushan Road-381, Guangzhou, 510641 China
These authors contributed equally to this work.
Search for more papers by this authorYubing Pang
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry & Chemical Engineering, South China University of Technology, Wushan Road-381, Guangzhou, 510641 China
These authors contributed equally to this work.
Search for more papers by this authorYuecheng Wu
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry & Chemical Engineering, South China University of Technology, Wushan Road-381, Guangzhou, 510641 China
Search for more papers by this authorNanping Zhang
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry & Chemical Engineering, South China University of Technology, Wushan Road-381, Guangzhou, 510641 China
Search for more papers by this authorRui Yang
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry & Chemical Engineering, South China University of Technology, Wushan Road-381, Guangzhou, 510641 China
Search for more papers by this authorYing Li
School of Biotechnology and Health Sciences, Wuyi University, Jiangmen, 529020 P. R. China
Search for more papers by this authorPengquan Chen
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry & Chemical Engineering, South China University of Technology, Wushan Road-381, Guangzhou, 510641 China
Search for more papers by this authorProf. Dr. Huanfeng Jiang
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry & Chemical Engineering, South China University of Technology, Wushan Road-381, Guangzhou, 510641 China
Search for more papers by this authorProf. Dr. Xue-Tao Xu
School of Biotechnology and Health Sciences, Wuyi University, Jiangmen, 529020 P. R. China
Search for more papers by this authorProf. Dr. Toh-Seok Kam
Department of Chemistry, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia
Search for more papers by this authorProf. Dr. Ting Fan
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry & Chemical Engineering, South China University of Technology, Wushan Road-381, Guangzhou, 510641 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhiqiang Ma
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry & Chemical Engineering, South China University of Technology, Wushan Road-381, Guangzhou, 510641 China
Search for more papers by this authorCheng Wang
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry & Chemical Engineering, South China University of Technology, Wushan Road-381, Guangzhou, 510641 China
These authors contributed equally to this work.
Search for more papers by this authorYubing Pang
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry & Chemical Engineering, South China University of Technology, Wushan Road-381, Guangzhou, 510641 China
These authors contributed equally to this work.
Search for more papers by this authorYuecheng Wu
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry & Chemical Engineering, South China University of Technology, Wushan Road-381, Guangzhou, 510641 China
Search for more papers by this authorNanping Zhang
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry & Chemical Engineering, South China University of Technology, Wushan Road-381, Guangzhou, 510641 China
Search for more papers by this authorRui Yang
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry & Chemical Engineering, South China University of Technology, Wushan Road-381, Guangzhou, 510641 China
Search for more papers by this authorYing Li
School of Biotechnology and Health Sciences, Wuyi University, Jiangmen, 529020 P. R. China
Search for more papers by this authorPengquan Chen
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry & Chemical Engineering, South China University of Technology, Wushan Road-381, Guangzhou, 510641 China
Search for more papers by this authorProf. Dr. Huanfeng Jiang
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry & Chemical Engineering, South China University of Technology, Wushan Road-381, Guangzhou, 510641 China
Search for more papers by this authorProf. Dr. Xue-Tao Xu
School of Biotechnology and Health Sciences, Wuyi University, Jiangmen, 529020 P. R. China
Search for more papers by this authorProf. Dr. Toh-Seok Kam
Department of Chemistry, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia
Search for more papers by this authorProf. Dr. Ting Fan
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry & Chemical Engineering, South China University of Technology, Wushan Road-381, Guangzhou, 510641 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhiqiang Ma
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry & Chemical Engineering, South China University of Technology, Wushan Road-381, Guangzhou, 510641 China
Search for more papers by this authorGraphical Abstract
Abstract
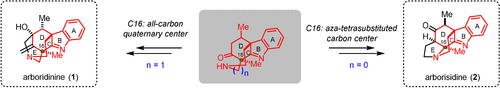
A divergent synthesis of skeletally distinct arboridinine and arborisidine was achieved. The central divergent strategy was inspired by the divergent biosynthetic cyclization mode of arboridinine and arborisidine and their hidden topological connection. The branch point was reached through a Michael and Mannich cascade process. A site-selective intramolecular Mannich reaction was developed to construct the tetracyclic core of arboridinine, while a site-selective intramolecular α-amination of ketone was used to access the tetracyclic core of arborisidine. A strategic Peterson olefination through intramolecular nucleophile delivery was able to set up the exocyclic olefin of arboridinine.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202110149-sup-0001-misc_information.pdf7.2 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. B. Herbert, In The Chemistry of Heterocyclic Compounds. Indoles Part 4, The Monoterpenoid Indole Alkaloids (Ed.: J. E. Saxton), Wiley, New York, 1983;
- 1bJ. Leonard, Nat. Prod. Rep. 1999, 16, 319–338;
- 1cS. E. O'Connor, J. J. Maresh, Nat. Prod. Rep. 2006, 23, 532–547;
- 1dM. Ishikura, K. Yamada, T. Abe, Nat. Prod. Rep. 2010, 27, 1630–1680;
- 1eM. Ishikura, T. Abe, T. Choshi, S. Hibino, Nat. Prod. Rep. 2013, 30, 694–752.
- 2For recent reviews on akuammiline alkaloid total synthesis, see:
- 2aR. Eckermann, T. Gaich, Synthesis 2013, 45, 2813–2823;
- 2bJ. M. Smith, J. Moreno, B. W. Boal, N. K. Garg, Angew. Chem. Int. Ed. 2015, 54, 400–412; Angew. Chem. 2015, 127, 410–422;
- 2cW. Zi, Z. Zuo, D. Ma, Acc. Chem. Res. 2015, 48, 702–711;
- 2dG. L. Adams, A. B. Smith III, The Chemistry of the Akuammiline Alkaloids, in The Alkaloids, Vol. 76 (Ed.: H.-J. Knolker), Elsevier, New York, 2016, pp. 171–257;
- 2eZ. Xu, Q. Wang, J. Zhu, Chem. Soc. Rev. 2018, 47, 7882–7898;
- 2fX.-Y. Liu, Y. Qin, Acc. Chem. Res. 2019, 52, 1877–1891.
- 3For recent reviews on strychnos alkaloid total synthesis, see:
- 3aM. Mori, Heterocycles 2010, 81, 259–292;
- 3bJ. S. Cannon, L. E. Overman, Angew. Chem. Int. Ed. 2012, 51, 4288–4311; Angew. Chem. 2012, 124, 4362–4386;
- 3cJ. M. Saya, E. Ruijter, R. V. A. Orru, Chem. Eur. J. 2019, 25, 8916–8935;
- 3dW. He, P. Wang, J. Chen, W. Xie, Org. Biomol. Chem. 2020, 18, 1046–1056.
- 4For recent reviews on aspidosperma alkaloid total synthesis, see:
- 4aB. P. Pritchett, B. M. Stoltz, Nat. Prod. Rep. 2018, 35, 559–574;
- 4bY. Wang, F. Xie, B. Lin, M. Cheng, Y. Liu, Chem. Eur. J. 2018, 24, 14302–14315.
- 5S.-P. Wong, C.-Y. Gan, K.-H. Lim, K.-N. Ting, Y.-Y. Low, T.-S. Kam, Org. Lett. 2015, 17, 3628–3631.
- 6S.-P. Wong, K.-W. Chong, K.-H. Lim, S.-H. Lim, Y.-Y. Low, T.-S. Kam, Org. Lett. 2016, 18, 1618–1621.
- 7H. Zhuang, S. Wei, Medicine composition for inhibiting stomach cancer. Chinese Patent CN106540237A, 2016.
- 8P. Gan, J. Pitzen, P. Qu, S. A. Snyder, J. Am. Chem. Soc. 2018, 140, 919–925.
- 9Z. Zhang, S. Xie, B. Cheng, H. Zhai, Y. Li, J. Am. Chem. Soc. 2019, 141, 7147–7154.
- 10Z. Chen, T. Xiao, H. Song, Y. Qin, Chin. J. Org. Chem. 2018, 38, 2427–2434.
- 11Z. Zhou, A. X. Gao, S. A. Snyder, J. Am. Chem. Soc. 2019, 141, 7715–7720.
- 12R. Andres, Q. Wang, J. Zhu, J. Am. Chem. Soc. 2020, 142, 14276–14285.
- 13F.-Y. Wang, L. Jiao, Angew. Chem. Int. Ed. 2021, 60, 12732–12736; Angew. Chem. 2021, 133, 12842–12846.
- 14
- 14aW. R. J. D. Galloway, A. Isidro-Llobet, D. R. Spring, Nat. Commun. 2010, 1, 80;
- 14bS. L. Schreiber, Science 2000, 287, 1964–1969;
- 14cL. Li, Z. Chen, X. Zhang, Y. Jia, Chem. Rev. 2018, 118, 3752–3832;
- 14dR. R. Merchant, K. M. Oberg, Y. Lin, A. J. E. Novak, J. Felding, P. S. Baran, J. Am. Chem. Soc. 2018, 140, 7462–7465;
- 14eC. J. Marth, G. M. Gallego, J. C. Lee, T. P. Lebold, S. Kulyk, K. G. M. Kou, J. Qin, R. Lilien, R. Sarpong, Nature 2015, 528, 493–498.
- 15
- 15aZ. Ma, X. Wang, X. Wang, R. A. Rodriguez, C. E. Moore, S. Gao, X. Tan, Y. Ma, A. L. Rheingold, P. S. Baran, C. Chen, Science 2014, 346, 219–224;
- 15bZ. Ma, X. Wang, Y. Ma, C. Chen, Angew. Chem. Int. Ed. 2016, 55, 4763–4766; Angew. Chem. 2016, 128, 4841–4844;
- 15cX. Wang, Z. Ma, J. Lu, X. Tan, C. Chen, J. Am. Chem. Soc. 2011, 133, 15350–15353.
- 16P. Chen, C. Wang, R. Yang, H. Xu, J. Wu, H. Jiang, K. Chen, Z. Ma, Angew. Chem. Int. Ed. 2021, 60, 5512–5518; Angew. Chem. 2021, 133, 5572–5578.
- 17K. Prantz, J. Mulzer, Chem. Rev. 2010, 110, 3741–3766.
- 18M. C. Fuente, D. Domínguez, Tetrahedron 2011, 67, 3997–4001.
- 19Z. Zhou, Q.-Q. Cheng, L. Kürti, J. Am. Chem. Soc. 2019, 141, 2242–2246.
- 20
- 20aP. K. Rabe, K. Kindler, Ber. Dtsch. Chem. Ges. 1918, 51, 466–467;
- 20bA. C. Smith, R. M. Williams, Angew. Chem. Int. Ed. 2008, 47, 1736–1740; Angew. Chem. 2008, 120, 1760–1764.
- 21A. S. Lee, B. B. Liau, M. D. Shair, J. Am. Chem. Soc. 2014, 136, 13442–13452.
- 22R. M. Moriarty, O. Prakash, I. Prakash, H. A. Musallam, J. Chem. Soc. Chem. Commun. 1984, 1342–1343.
- 23J. Strehl, G. Hilt, Org. Lett. 2020, 22, 5968–5972.
- 24P. Mizar, T. Wirth, Angew. Chem. Int. Ed. 2014, 53, 5993–5997; Angew. Chem. 2014, 126, 6103–6107.
- 25G. M. Kiefl, T. Gulder, J. Am. Chem. Soc. 2020, 142, 20577–20582.
- 26For selected examples of the synthesis of aza-trisubstituted carbon centers, see:
- 26aL. M. Kreis, E. M. Carreira, Angew. Chem. Int. Ed. 2012, 51, 3436–3439; Angew. Chem. 2012, 124, 3492–3495;
- 26bJ. S. Clark, C. Xu, Angew. Chem. Int. Ed. 2016, 55, 4332–4335; Angew. Chem. 2016, 128, 4404–4407;
- 26cW. Ren, Q. Wang, J. Zhu, Angew. Chem. Int. Ed. 2016, 55, 3500–3503; Angew. Chem. 2016, 128, 3561–3564; for one example of a synthesis of aza-tetrasubstituted carbon centers from an α-ketol precursor, see:
- 26dH. Li, X. Wang, X. Lei, Angew. Chem. Int. Ed. 2012, 51, 491–495; Angew. Chem. 2012, 124, 506–510; for a Minireview on the α-amination of carbonyl groups with nitrogen nucleophiles, see:
- 26eA. de la Torre, V. Tona, N. Maulide, Angew. Chem. Int. Ed. 2017, 56, 12416–12423; Angew. Chem. 2017, 129, 12588–12596.
- 27B.-C. Chen, P. Zhou, F. A. Davis, E. Ciganek, Org. React. 2003, 62, 1–356.
- 28For reviews on the pinacol rearrangement, see:
- 28aC. J. Collins, Q. Rev. Chem. Soc. 1960, 14, 357–377;
- 28bB. Rickborn, In Comprehensive Organic Synthesis (Eds.: B. M. Trost, I. Fleming), Pergamon, Oxford, U. K., 1991, pp. 721–732;
10.1016/B978-0-08-052349-1.00078-0 Google Scholar
- 28cZ.-L. Song, C.-A. Fan, Y.-Q. Tu, Chem. Rev. 2011, 111, 7523–7556.
- 29A. G. Kravina, E. M. Carreira, Angew. Chem. Int. Ed. 2018, 57, 13159–13162; Angew. Chem. 2018, 130, 13343–13346.
- 30For reviews on silicon-tethered reactions, see:
- 30aM. Bols, T. Skrydstrup, Chem. Rev. 1995, 95, 1253–1277;
- 30bS. Bracegirdle, E. A. Anderson, Chem. Soc. Rev. 2010, 39, 4114–4129.
- 31For reviews on the Peterson olefination, see:
- 31aD. J. Ager, Synthesis 1984, 384–398;
- 31bA. G. M. Barrett, J. M. Hill, E. M. Wallace, J. A. Flygare, Synlett 1991, 764–770;
- 31cL. F. van Staden, D. Gravestock, D. J. Ager, Chem. Soc. Rev. 2002, 31, 195–200;
- 31dP. R. Blakemore, Olefination of Carbonyl Compounds by Main Group Element Mediators, In Comprehensive Organic Synthesis, Vol. 1, 2nd ed. (Eds.: G. A. Molander, P. Knochel), Elsevier Ltd.) Oxford, 2014, pp. 516–608.
- 32S. R. Chemler, D. Trauner, S. J. Danishefsky, Angew. Chem. Int. Ed. 2001, 40, 4544–4568;
10.1002/1521-3773(20011217)40:24<4544::AID-ANIE4544>3.0.CO;2-N CAS PubMed Web of Science® Google ScholarAngew. Chem. 2001, 113, 4676–4701.
- 33Deposition Numbers 2056918 (20 a), 2056917 (20 c), 2110887 (26), 2056921 (28), 2056922 (34), 2056923 (1), 2056924 (35), 2056925 (37), and 2056926 (2) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.