Spatial Structure Regulation: A Rod-Shaped Viologen Enables Long Lifetime in Aqueous Redox Flow Batteries
Graphical Abstract
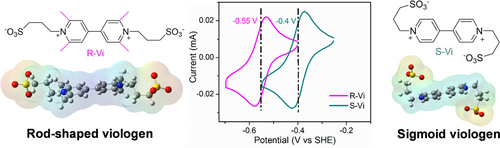
We report a rod-like sulfonated viologen (R-Vi) anolyte for aqueous redox flow batteries (AORFBs). R-Vi possesses a low membrane-penetration rate of ≈10−10 cm2 s−1 and the lowest redox potential of −0.55 V vs. SHE among one-electron-storage viologens in AORFBs simultaneously. The R-Vi-based AORFB exhibits an extremely low capacity-fade rate of 0.007 % per cycle in 3200 continuous charging–discharging processes.
Abstract
A stable rod-like sulfonated viologen (R-Vi) derivative is developed through a spatial-structure-adjustment strategy for neutral aqueous organic redox flow batteries (AORFBs). The obtained R-Vi features four individual methyl groups on the 2,2′,6,6′-positions of the 4,4′-bipyridine core ring. The tethered methyls confine the movement of the alkyl chain as well as the sulfonic anion, thus driving the spatial structure from sigmoid to rod shape. The R-Vi with weak charge attraction and large molecular dimension displays an ultralow membrane permeability that is only 14.7 % of that of typical sigmoid viologen. Moreover, the electron-donating effect of methyls endows R-Vi with the lowest redox potential of −0.55 V vs. SHE among one-electron-storage viologen-based AORFBs. The AORFB with the R-Vi anolyte and a K4Fe(CN)6 catholyte exhibits an energy efficiency up to 87 % and extremely low capacity-fade rate of 0.007 % per cycle in 3200 continuous cycles.
Conflict of interest
The authors declare no conflict of interest.