Enzyme Catalysis in Non-Native Environment with Unnatural Selectivity Using Polymeric Nanoreactors
Dr. Jingjing Gao
Department of Chemistry, University of Massachusetts Amherst, Amherst, MA, 01003 USA
Center for Nanomedicine and Division of Engineering in Medicine, Department of Anesthesiology, Brigham and Women's Hospital, Boston, MA, 02115 USA
Harvard Medical School, Boston, MA, 02115 USA
Search for more papers by this authorStephanie Le
Department of Chemistry, University of Massachusetts Amherst, Amherst, MA, 01003 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. S. Thayumanavan
Department of Chemistry, University of Massachusetts Amherst, Amherst, MA, 01003 USA
Search for more papers by this authorDr. Jingjing Gao
Department of Chemistry, University of Massachusetts Amherst, Amherst, MA, 01003 USA
Center for Nanomedicine and Division of Engineering in Medicine, Department of Anesthesiology, Brigham and Women's Hospital, Boston, MA, 02115 USA
Harvard Medical School, Boston, MA, 02115 USA
Search for more papers by this authorStephanie Le
Department of Chemistry, University of Massachusetts Amherst, Amherst, MA, 01003 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. S. Thayumanavan
Department of Chemistry, University of Massachusetts Amherst, Amherst, MA, 01003 USA
Search for more papers by this authorGraphical Abstract
Abstract
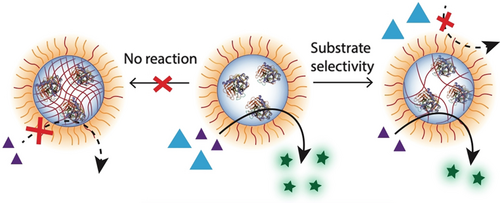
The utilization of enzymes for catalysis in organic solvents, while exhibiting selectivity to different substrates, is a big challenge. We report an amphiphilic random copolymer system that self-assembles with enzymes in an organic solvent to form nanoreactors. These encapsulated enzymes are not denatured and they do preserve the catalytic activity. The cross-linkable functional groups in the hydrophobic compartments of the polymers offer to control accessibility to the enzyme. This varied accessibility due to the polymer host, rather than the enzyme itself, endows the nanoreactor with an unnatural selectivity. The findings here highlight the significant potential of simple polymer-based enzyme nanoreactors to execute selective organic reactions under non-native conditions.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202109477-sup-0001-misc_information.pdf1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. Cornish-Bowden, A. Cornish-Bowden, Fundamentals of Enzyme Kinetics, Vol. 510, Wiley-Blackwell, Weinheim, 2012.
- 2A. Fersht, Enzyme Structure, and Mechanism, Vol. 99, W. H. Freeman, New York, 1985.
- 3C. Grondal, M. Jeanty, D. Enders, Nat. Chem. 2010, 2, 167.
- 4S. Schoffelen, J. C. M. van Hest, Curr. Opin. Struct. Biol. 2013, 23, 613–621.
- 5Y. Wang, H. Lu, P.-F. Xu, Acc. Chem. Res. 2015, 48, 1832–1844.
- 6D. E. Atkinson, Annu. Rev. Microbiol. 1969, 23, 47–68.
- 7P. V. Iyer, L. Ananthanarayan, Process Biochem. 2008, 43, 1019–1032.
- 8G. Parkin, Chem. Rev. 2004, 104, 699–768.
- 9W. A. Duetz, J. B. Van Beilen, B. Witholt, Curr. Opin. Biotechnol. 2001, 12, 419–425.
- 10P. Asuri, S. S. Karajanagi, H. Yang, T.-J. Yim, R. S. Kane, J. S. Dordick, Langmuir 2006, 22, 5833–5836.
- 11K. A. Dill, Biochemistry 1990, 29, 7133–7155.
- 12M. N. Gupta, I. Roy, Eur. J. Biochem. 2004, 271, 2575–2583.
- 13X. Wang, J. Hu, G. Liu, J. Tian, H. Wang, M. Gong, S. Liu, J. Am. Chem. Soc. 2015, 137, 15262–15275.
- 14Z. Deng, Y. Qian, Y. Yu, G. Liu, J. Hu, G. Zhang, S. Liu, J. Am. Chem. Soc. 2016, 138, 10452–10466.
- 15A. J. Miller, A. K. Pearce, J. C. Foster, R. K. O'Reilly, ACS Cent. Sci. 2021, 7, 30–38.
- 16X. Liu, D. Appelhans, B. Voit, J. Am. Chem. Soc. 2018, 140, 16106–16114.
- 17L. D. Blackman, S. Varlas, M. C. Arno, Z. H. Houston, N. L. Fletcher, K. J. Thurecht, M. Hasan, M. I. Gibson, R. K. O'Reilly, ACS Cent. Sci. 2018, 4, 718–723.
- 18X. Yang, Q. Tang, Y. Jiang, M. Zhang, M. Wang, L. Mao, J. Am. Chem. Soc. 2019, 141, 3782–3786.
- 19R. J. Kazlauskas, U. T. Bornscheuer, Nat. Chem. Biol. 2009, 5, 526.
- 20M. B. Quin, C. Schmidt-Dannert, ACS Catal. 2011, 1, 1017–1021.
- 21S. Lutz, Curr. Opin. Biotechnol. 2010, 21, 734–743.
- 22P.-S. Huang, S. E. Boyken, D. Baker, Nature 2016, 537, 320–327.
- 23S. Lutz, W. M. Patrick, Curr. Opin. Biotechnol. 2004, 15, 291–297.
- 24J. A. Rodríguez-Martínez?, R. J. Solá, B. Castillo, H. R. Cintrón-Colón, I. Rivera-Rivera, G. Barletta, K. Griebenow, Biotechnol. Bioeng. 2008, 101, 1142–1149.
- 25B. Castillo, R. J. Solá, A. Ferrer, G. Barletta, K. Griebenow, Biotechnol. Bioeng. 2008, 99, 9–17.
- 26F. M. Veronese, R. Largajolli, E. Boccu, C. A. Benassi, O. Schiavon, Appl. Biochem. Biotechnol. 1985, 11, 141–152.
- 27Y. Tamaura, K. Takahashi, Y. Kodera, Y. Saito, Y. Inada, Biotechnol. Lett. 1986, 8, 877–880.
- 28D. Avnir, S. Braun, O. Lev, M. Ottolenghi, Chem. Mater. 1994, 6, 1605–1614.
- 29P. Tielmann, H. Kierkels, A. Zonta, A. Ilie, M. T. Reetz, Nanoscale 2014, 6, 6220–6228.
- 30S. Braun, S. Rappoport, R. Zusman, D. Avnir, M. Ottolenghi, Mater. Lett. 1990, 10, 1–5.
- 31S. Chakraborty, R. Khamrui, S. Ghosh, Chem. Sci. 2021, 12, 1101–1108.
- 32Y. Bai, Q. Luo, J. Liu, Chem. Soc. Rev. 2016, 45, 2756–2767.
- 33M. Zoumpanioti, M. Karali, A. Xenakis, H. Stamatis, Enzyme Microb. Technol. 2006, 39, 531–539.
- 34E. Mitsou, A. Xenakis, M. Zoumpanioti, Catalysts 2017, 7, 52.
- 35H. Renata, Z. J. Wang, F. H. Arnold, Angew. Chem. Int. Ed. 2015, 54, 3351–3367; Angew. Chem. 2015, 127, 3408–3426.
- 36A. Klibanov, Nature 2001, 409, 241–246.
- 37H. C. Yocum, A. Pham, N. A. Da Silva, Front. Bioeng. Biotechnol. 2021, 9, 37.
- 38M. Moniruzzaman, N. Kamiya, K. Nakashima, M. Goto, Green Chem. 2008, 10, 497–500.
- 39V. R. Vasquez, B. C. Williams, O. A. Graeve, J. Phys. Chem. B 2011, 115, 2979–2987.
- 40F. D. Souza, B. S. Souza, D. W. Tondo, E. C. Leopoldino, H. D. Fiedler, F. Nome, Langmuir 2015, 31, 3587–3595.
- 41J. Gao, B. Zhao, M. Wang, M. A. C. Serrano, J. Zhuang, M. Ray, V. M. Rotello, R. W. Vachet, S. Thayumanavan, J. Am. Chem. Soc. 2018, 140, 2421–2425.
- 42B. Panganiban, B. Qiao, T. Jiang, C. DelRe, M. M. Obadia, T. D. Nguyen, A. A. A. Smith, A. Hall, I. Sit, M. G. Crosby, P. B. Dennis, E. Drockenmuller, M. O. De La Cruz, T. Xu, Science 2018, 359, 1239–1243.
- 43B. Zhao, J. Gao, M. A. C. Serrano, K. F. Arcaro, S. Thayumanavan, R. W. Vachet, Anal. Bioanal. Chem. 2020, 412, 1027–1035.
- 44M. A. C. Serrano, J. Gao, K. A. Kelly, S. Thayumanavan, R. W. Vachet, ACS Appl. Mater. Interfaces 2018, 10, 40443–40451.
- 45T. D. Nguyen, B. Qiao, M. O. De La Cruz, Proc. Natl. Acad. Sci. USA 2018, 115, 6578–6583.
- 46J. Gao, K. Dutta, J. Zhuang, S. Thayumanavan, Angew. Chem. Int. Ed. 2020, 59, 23466–23470; Angew. Chem. 2020, 132, 23672–23676.
- 47M. Wang, J. Gao, B. Zhao, S. Thayumanavan, R. W. Vachet, Analyst 2019, 144, 6321–6326.
- 48J. Gao, H. Wang, J. Zhuang, S. Thayumanavan, Chem. Sci. 2019, 10, 3018–3024.