A Molecular Compound for Highly Selective Purification of Ethylene
Anurag Noonikara-Poyil
Department of Chemistry and Biochemistry, The University of Texas at Arlington, Arlington, TX, 76019 USA
These authors contributed equally to this work.
Search for more papers by this authorHui Cui
Department of Chemistry, University of Texas at San Antonio, San Antonio, TX, 78249 USA
These authors contributed equally to this work.
Search for more papers by this authorDr. Andrey A. Yakovenko
X-Ray Science Division, Advanced Photon Source, Argonne National Laboratory, Argonne, IL, 60439 USA
Search for more papers by this authorProf. Dr. Peter W. Stephens
Department of Physics and Astronomy, Stony Brook University, Stony Brook, NY, 11794-3800 USA
Search for more papers by this authorProf. Dr. Rui-Biao Lin
MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorBin Wang
Department of Chemistry, University of Texas at San Antonio, San Antonio, TX, 78249 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Banglin Chen
Department of Chemistry, University of Texas at San Antonio, San Antonio, TX, 78249 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. H. V. Rasika Dias
Department of Chemistry and Biochemistry, The University of Texas at Arlington, Arlington, TX, 76019 USA
Search for more papers by this authorAnurag Noonikara-Poyil
Department of Chemistry and Biochemistry, The University of Texas at Arlington, Arlington, TX, 76019 USA
These authors contributed equally to this work.
Search for more papers by this authorHui Cui
Department of Chemistry, University of Texas at San Antonio, San Antonio, TX, 78249 USA
These authors contributed equally to this work.
Search for more papers by this authorDr. Andrey A. Yakovenko
X-Ray Science Division, Advanced Photon Source, Argonne National Laboratory, Argonne, IL, 60439 USA
Search for more papers by this authorProf. Dr. Peter W. Stephens
Department of Physics and Astronomy, Stony Brook University, Stony Brook, NY, 11794-3800 USA
Search for more papers by this authorProf. Dr. Rui-Biao Lin
MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorBin Wang
Department of Chemistry, University of Texas at San Antonio, San Antonio, TX, 78249 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Banglin Chen
Department of Chemistry, University of Texas at San Antonio, San Antonio, TX, 78249 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. H. V. Rasika Dias
Department of Chemistry and Biochemistry, The University of Texas at Arlington, Arlington, TX, 76019 USA
Search for more papers by this authorGraphical Abstract
Abstract
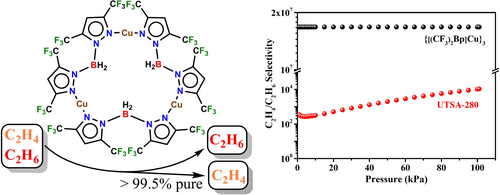
Purification of C2H4 from an C2H4 /C2H6 mixture is one of the most challenging separation processes, which is achieved mainly through energy-intensive, cryogenic distillation in industry. Sustainable, non-distillation methods are highly desired as alternatives. We discovered that the fluorinated bis(pyrazolyl)borate ligand supported copper(I) complex {[(CF3)2Bp]Cu}3 has features very desirable in an olefin–paraffin separation material. It binds ethylene exclusively over ethane generating [(CF3)2Bp]Cu(C2H4). This molecular compound exhibits extremely high and record ideal adsorbed solution theory (IAST) C2H4 /C2H6 gas separation selectivity, affording high purity (>99.5 %) ethylene that can be readily desorbed from separation columns. In-situ PXRD provides a “live” picture of the reversible conversion between [(CF3)2Bp]Cu(C2H4) and the ethylene-free sorbent in the solid-state, driven by the presence or removal of C2H4. Molecular structures of trinuclear {[(CF3)2Bp]Cu}3 and mononuclear [(CF3)2Bp]Cu(C2H4) are also presented.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202109338-sup-0001-cif.zip837.3 KB | Supporting Information |
| anie202109338-sup-0001-misc_information.pdf2.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. S. Sholl, R. P. Lively, Nature 2016, 532, 435–437;
- 1bS. Chu, N. Liu, S. Chu, Y. Cui, N. Liu, Y. Cui, Nat. Mater. 2017, 16, 16–22.
- 2
- 2aH. Li, L. Li, R.-B. Lin, W. Zhou, Z. Zhang, S. Xiang, B. Chen, EnergyChem 2019, 1, 100006;
10.1016/j.enchem.2019.100006 Google Scholar
- 2bR.-B. Lin, S. Xiang, W. Zhou, B. Chen, Chem 2020, 6, 337–363.
- 3
- 3aE. D. Bloch, W. L. Queen, R. Krishna, J. M. Zadrozny, C. M. Brown, J. R. Long, Science 2012, 335, 1606–1610;
- 3bY. He, R. Krishna, B. Chen, Energy Environ. Sci. 2012, 5, 9107–9120;
- 3cS. Yang, A. J. Ramirez-Cuesta, R. Newby, V. Garcia-Sakai, P. Manuel, S. K. Callear, S. I. Campbell, C. C. Tang, M. Schroder, Nat. Chem. 2015, 7, 121–129;
- 3dB. Li, Y. Zhang, R. Krishna, K. Yao, Y. Han, Z. Wu, D. Ma, Z. Shi, T. Pham, B. Space, J. Liu, P. K. Thallapally, J. Liu, M. Chrzanowski, S. Ma, J. Am. Chem. Soc. 2014, 136, 8654–8660;
- 3eS. Aguado, G. Bergeret, C. Daniel, D. Farrusseng, J. Am. Chem. Soc. 2012, 134, 14635–14637;
- 3fP. J. Bereciartua, Á. Cantín, A. Corma, J. L. Jordá, M. Palomino, F. Rey, S. Valencia, E. W. Corcoran, P. Kortunov, P. I. Ravikovitch, A. Burton, C. Yoon, Y. Wang, C. Paur, J. Guzman, A. R. Bishop, G. L. Casty, Science 2017, 358, 1068–1071;
- 3gL. Li, R.-B. Lin, R. Krishna, H. Li, S. Xiang, H. Wu, J. Li, W. Zhou, B. Chen, Science 2018, 362, 443–446;
- 3hR.-B. Lin, L. Li, H.-L. Zhou, H. Wu, C. He, S. Li, R. Krishna, J. Li, W. Zhou, B. Chen, Nat. Mater. 2018, 17, 1128–1133.
- 4B. M. Binder, J. Biol. Chem. 2020, 295, 7710–7725.
- 5
- 5aF. I. Rodríguez, J. J. Esch, A. E. Hall, B. M. Binder, G. E. Schaller, A. B. Bleeckert, Science 1999, 283, 996–998;
- 5bG. E. Schaller, A. B. Bleecker, Science 1995, 270, 1809–1811;
- 5cS. Schott-Verdugo, L. Müller, E. Classen, H. Gohlke, G. Groth, Sci. Rep. 2019, 9, 8869.
- 6S. Trofimenko, Chem. Rev. 1993, 93, 943–980.
- 7
- 7aJ. S. Thompson, R. L. Harlow, J. F. Whitney, J. Am. Chem. Soc. 1983, 105, 3522–3527;
- 7bH. V. R. Dias, C. J. Lovely, Chem. Rev. 2008, 108, 3223–3238.
- 8
- 8aM. Munakata, S. Kitagawa, S. Kosome, A. Asahara, Inorg. Chem. 1986, 25, 2622–2627;
- 8bB. F. Straub, F. Eisentrager, P. Hofmann, Chem. Commun. 1999, 2507–2508.
- 9H. V. R. Dias, H.-L. Lu, H.-J. Kim, S. A. Polach, T. K. H. H. Goh, R. G. Browning, C. J. Lovely, Organometallics 2002, 21, 1466–1473.
- 10
- 10aD. Parasar, A. H. Elashkar, A. A. Yakovenko, N. B. Jayaratna, B. L. Edwards, S. G. Telfer, H. V. R. Dias, M. G. Cowan, Angew. Chem. Int. Ed. 2020, 59, 21001–21006; Angew. Chem. 2020, 132, 21187–21192;
- 10bN. B. Jayaratna, M. G. Cowan, D. Parasar, H. H. Funke, J. Reibenspies, P. K. Mykhailiuk, O. Artamonov, R. D. Noble, H. V. R. Dias, Angew. Chem. Int. Ed. 2018, 57, 16442–16446; Angew. Chem. 2018, 130, 16680–16684;
- 10cM. G. Cowan, W. M. McDanel, H. H. Funke, Y. Kohno, D. L. Gin, R. D. Noble, Angew. Chem. Int. Ed. 2015, 54, 5740–5743; Angew. Chem. 2015, 127, 5832–5835.
- 11H. V. R. Dias, S. A. Richey, H. V. K. Diyabalanage, J. Thankamani, J. Organomet. Chem. 2005, 690, 1913–1922.
- 12E. Maslowsky, Jr., Vibrational Spectra of Organometallics: Theoretical and Experimental Data, Wiley, Hoboken, 2019.
- 13Deposition Numbers 2088726 (for 3) and 2088727 (for 4) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 14G. Mezei, C. M. Zaleski, V. L. Pecoraro, Chem. Rev. 2007, 107, 4933–5003.
- 15C. R. Groom, I. J. Bruno, M. P. Lightfoot, S. C. Ward, Acta Crystallogr. Sect. B 2016, 72, 171–179.
- 16S. J. Geier, J. A. Mason, E. D. Bloch, W. L. Queen, M. R. Hudson, C. M. Brown, J. R. Long, Chem. Sci. 2013, 4, 2054–2061.
- 17L. Zhang, L. Li, E. Hu, L. Yang, K. Shao, L. Yao, K. Jiang, Y. Cui, Y. Yang, B. Li, B. Chen, G. Qian, Adv. Sci. 2020, 7, 1901918.
- 18Z. Bao, J. Wang, Z. Zhang, H. Xing, Q. Yang, Y. Yang, H. Wu, R. Krishna, W. Zhou, B. Chen, Q. Ren, Angew. Chem. Int. Ed. 2018, 57, 16020–16025; Angew. Chem. 2018, 130, 16252–16257.
- 19P. Li, Y. He, H. D. Arman, R. Krishna, H. Wang, L. Weng, B. Chen, Chem. Commun. 2014, 50, 13081–13084.
- 20R. Faiz, K. Li, Chem. Eng. Sci. 2012, 73, 261–284.
- 21B. Claessens, G. R. Wittevrongel, F. Rey, S. Valencia, J. Cousin-Saint-Remi, G. V. Baron, J. F. M. Denayer, Chem. Eng. J. 2021, 412, 128658.
- 22S. Van der Perre, P. Gelin, B. Claessens, A. Martin-Calvo, J. Cousin Saint Remi, T. Duerinck, G. V. Baron, M. Palomino, L. Y. Sánchez, S. Valencia, J. Shang, R. Singh, P. A. Webley, F. Rey, J. F. M. Denayer, ChemSusChem 2017, 10, 2968–2977.