Fluorinated S-Adenosylmethionine as a Reagent for Enzyme-Catalyzed Fluoromethylation
Jiaming Peng
Department of Chemistry, University of Basel, Mattenstrasse 24a, 4002 Basel, Switzerland
These authors contributed equally to this work.
Search for more papers by this authorCangsong Liao
Department of Chemistry, University of Basel, Mattenstrasse 24a, 4002 Basel, Switzerland
These authors contributed equally to this work.
Search for more papers by this authorCarsten Bauer
Department of Chemistry, University of Basel, Mattenstrasse 24a, 4002 Basel, Switzerland
Search for more papers by this authorCorresponding Author
Florian P. Seebeck
Department of Chemistry, University of Basel, Mattenstrasse 24a, 4002 Basel, Switzerland
Search for more papers by this authorJiaming Peng
Department of Chemistry, University of Basel, Mattenstrasse 24a, 4002 Basel, Switzerland
These authors contributed equally to this work.
Search for more papers by this authorCangsong Liao
Department of Chemistry, University of Basel, Mattenstrasse 24a, 4002 Basel, Switzerland
These authors contributed equally to this work.
Search for more papers by this authorCarsten Bauer
Department of Chemistry, University of Basel, Mattenstrasse 24a, 4002 Basel, Switzerland
Search for more papers by this authorCorresponding Author
Florian P. Seebeck
Department of Chemistry, University of Basel, Mattenstrasse 24a, 4002 Basel, Switzerland
Search for more papers by this authorGraphical Abstract
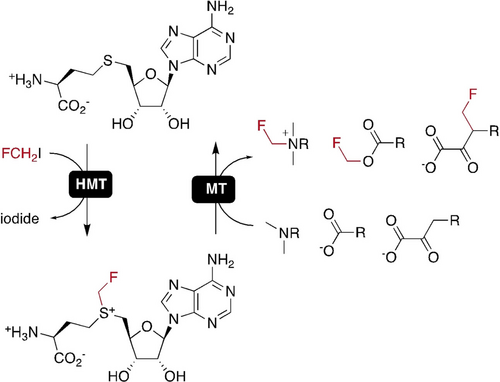
In a biocatalytic approach to the production of fluorinated compounds, halide methyltransferases (HMTs) were found to form fluorinated S-adenosylmethionine (SAM) from S-adenosylhomocysteine and fluoromethyliodide. Although too unstable for isolation, fluorinated SAM was accepted as a substrate by C-, N- and O-specific SAM-dependent methyltransferases (MTs) to enable the fluoromethylation of small molecules (see scheme).
Abstract
Strategic replacement of protons with fluorine atoms or functional groups with fluorine-containing fragments has proven a powerful strategy to optimize the activity of therapeutic compounds. For this reason, the synthetic chemistry of organofluorides has been the subject of intense development and innovation for many years. By comparison, the literature on fluorine biocatalysis still makes for a slim chapter. Herein we introduce S-adenosylmethionine (SAM) dependent methyltransferases as a new tool for the production of fluorinated compounds. We demonstrate the ability of halide methyltransferases to form fluorinated SAM (S-adenosyl-S-(fluoromethyl)-L-homocysteine) from S-adenosylhomocysteine and fluoromethyliodide. Fluorinated SAM (F-SAM) is too unstable for isolation, but is accepted as a substrate by C-, N- and O-specific methyltransferases for enzyme-catalyzed fluoromethylation of small molecules.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202108802-sup-0001-misc_information.pdf4.9 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aL. Hunter, Beilstein J. Org. Chem. 2010, 6, 38;
- 1bM. Aufiero, R. Gilmour, Acc. Chem. Res. 2018, 51, 1701–1710;
- 1cY. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J. L. Aceña, V. A. Soloshonok, K. Izawa, H. Liu, Chem. Rev. 2016, 116, 422–518;
- 1dS. K. Holmgren, K. M. Taylor, L. E. Bretscher, R. T. Raines, Nature 1998, 392, 666–667;
- 1eL. Merkel, N. Budisa, Org. Biomol. Chem. 2012, 10, 7241–7261;
- 1fW. Zhu, P. F. Doubleday, D. S. Catlin, P. M. Weerawarna, A. Butrin, S. Shen, Z. Wawrzak, N. L. Kelleher, D. Liu, R. B. Silverman, J. Am. Chem. Soc. 2020, 142, 4892–4903.
- 2
- 2aP. W. Miller, N. J. Long, R. Vilar, A. D. Gee, Angew. Chem. Int. Ed. 2008, 47, 8998–9033; Angew. Chem. 2008, 120, 9136–9172;
- 2bD. van der Born, A. Pees, A. J. Poot, R. V. A. Orru, A. D. Windhorst, D. J. Vugts, Chem. Soc. Rev. 2017, 46, 4709–4773;
- 2cR. A. Werner, T. Derlin, C. Lapa, S. Sheikbahaei, T. Higuchi, F. L. Giesel, S. Behr, A. Drzezga, H. Kimura, A. K. Buck, F. M. Bengel, M. G. Pomper, M. A. Gorin, S. P. Rowe, Theranostics 2020, 10, 1–16.
- 3R. Szpera, D. F. J. Moseley, L. B. Smith, A. J. Sterling, V. Gouverneur, Angew. Chem. Int. Ed. 2019, 58, 14824–14848; Angew. Chem. 2019, 131, 14966–14991.
- 4
- 4aM. Reichel, K. Karaghiosoff, Angew. Chem. Int. Ed. 2020, 59, 12268–12281; Angew. Chem. 2020, 132, 12364–12377;
- 4bG. Parisi, M. Colella, S. Monticelli, G. Romanazzi, W. Holzer, T. Langer, L. Degennaro, V. Pace, R. Luisi, J. Am. Chem. Soc. 2017, 139, 13648–13651;
- 4cT. Ding, L. Jiang, J. Yang, Y. Xu, G. Wang, W. Yi, Org. Lett. 2019, 21, 6025–6028;
- 4dG. K. Prakash, S. Chacko, S. Alconcel, T. Stewart, T. Mathew, G. A. Olah, Angew. Chem. Int. Ed. 2007, 46, 4933–4936; Angew. Chem. 2007, 119, 5021–5024;
- 4eT. Scattolin, S. Bouayad-Gervais, F. Schoenebeck, Nature 2019, 573, 102–107;
- 4fT. Koike, M. Akita, Acc. Chem. Res. 2016, 49, 1937–1945;
- 4gJ. Charpentier, N. Früh, A. Togni, Chem. Rev. 2015, 115, 650–682;
- 4hJ. W. Lee, K. N. Lee, M. Y. Ngai, Angew. Chem. Int. Ed. 2019, 58, 11171–11181; Angew. Chem. 2019, 131, 11289–11299;
- 4iS. L. Clarke, G. P. McGlacken, Chem. Eur. J. 2017, 23, 1219–1230;
- 4jV. Fasano, N. Winter, A. Noble, V. K. Aggarwal, Angew. Chem. Int. Ed. 2020, 59, 8502–8506; Angew. Chem. 2020, 132, 8580–8584;
- 4kJ. Moschner, V. Stulberg, R. Fernandes, S. Huhmann, J. Leppkes, B. Koksch, Chem. Rev. 2019, 119, 10718–10801.
- 5
- 5aH. Zheng, Z. Li, J. Jing, X. S. Xue, J. P. Cheng, Angew. Chem. Int. Ed. 2021, 60, 9401–9406; Angew. Chem. 2021, 133, 9487–9492;
- 5bR. Reichel, S. Sile, A. Kornath, K. Karaghiosoff, J. Org. Chem. 2021, 86, 4423–4431;
- 5cJ. Sheng, H. Q. Ni, S. X. Ni, Y. He, R. Cui, G. X. Liao, K. J. Bian, B. B. Wu, X. S. Wang, Angew. Chem. Int. Ed. 2021, 60, 15020–15027; Angew. Chem. 2021, 133, 15147–15154.
- 6
- 6aD. O'Hagan, H. Deng, Chem. Rev. 2015, 115, 634–649;
- 6bL. Wu, F. Maglangit, H. Deng, Curr. Opin. Chem. Biol. 2020, 55, 119–126.
- 7M. F. Carvalho, R. S. Oliveira, Crit. Rev. Biotechnol. 2017, 37, 880–897.
- 8K. Markakis, P. T. Lowe, L. Davison-Gates, D. O'Hagan, S. J. Rosser, A. Elfick, ChemBioChem 2020, 21, 1856–1860.
- 9
- 9aX. Huang, M. Garcia-Borràs, K. Miao, S. B. J. Kan, A. Zutshi, K. N. Houk, F. H. Arnold, ACS Cent. Sci. 2019, 5, 270–276;
- 9bJ. Fang, D. Hait, M. Head-Gordon, M. C. Y. Chang, Angew. Chem. Int. Ed. 2019, 58, 11841–11845; Angew. Chem. 2019, 131, 11967–11971;
- 9cH. Sun, W. L. Yeo, Y. H. Lim, X. Chew, D. J. Smith, B. Xue, K. P. Chan, R. C. Robinson, E. G. Robins, H. Zhao, E. L. Ang, Angew. Chem. Int. Ed. 2016, 55, 14277–14280; Angew. Chem. 2016, 128, 14489–14492;
- 9dD. M. Carminati, J. Decaens, S. Couve-Bonnaire, P. Jubault, R. Fasan, Angew. Chem. Int. Ed. 2021, 60, 7072–7076; Angew. Chem. 2021, 133, 7148–7152.
- 10
- 10aC. Liao, F. P. Seebeck, Nat. Catal. 2019, 2, 696–701;
- 10bC. Liao, F. P. Seebeck, Angew. Chem. Int. Ed. 2020, 59, 7184–7187; Angew. Chem. 2020, 132, 7251–7254;
- 10cI. J. W. McKean, P. A. Hoskisson, G. A. Burley, ChemBioChem 2020, 21, 2890–2897.
- 11
- 11aP. Schneider, B. Henßen, B. Paschold, B. P. Chapple, M. Schatton, F. Seebeck, T. Classen, J. Pietruszka, Angew. Chem. Int. Ed. 2021, 60, 23412–23418; Angew. Chem. 2021, 133, 23600–23606;
- 11bM. A. Beliaeva, R. Burn, D. Lim, F. P. Seebeck, Angew. Chem. Int. Ed. 2021, 60, 5209–5212; Angew. Chem. 2021, 133, 5269–5272.
- 12
- 12aQ. Tang, C. W. Grathwol, A. S. Aslan-Üzel, S. Wu, A. Link, I. V. Pavlidis, C. P. S. Badenhorst, U. T. Bornscheuer, Angew. Chem. Int. Ed. 2021, 60, 1524–1527; Angew. Chem. 2021, 133, 1547–1551;
- 12bL. L. Bengel, B. Aberle, A. N. Egler-Kemmerer, S. Kienzle, B. Hauer, S. C. Hammer, Angew. Chem. Int. Ed. 2021, 60, 5554–55560.
- 13A. J. Herbert, S. A. Shepherd, V. A. Cronin, M. R. Bennett, R. Sung, J. Micklefield, Angew. Chem. Int. Ed. 2020, 59, 14950–14956; Angew. Chem. 2020, 132, 15060–15066.
- 14F. Michailidou, A. Rentmeister, Org. Biomol. Chem. 2021, 19, 3756–3762.
- 15D. F. Iwig, S. J. Booker, Biochemistry 2004, 43, 13496–13509.
- 16
- 16aW. Ding, Y. Li, J. Zhao, X. Ji, T. Mo, H. Qianzhu, T. Tu, Z. Deng, Y. Yu, F. Chen, Q. Zhang, Angew. Chem. Int. Ed. 2017, 56, 3857–3861; Angew. Chem. 2017, 129, 3915–3919;
- 16bX. Ji, D. Mandalapu, J. Cheng, W. Ding, Q. Zhang, Angew. Chem. Int. Ed. 2018, 57, 6601–6604; Angew. Chem. 2018, 130, 6711–6714.
- 17A. S. Eustáquio, J. Härle, J. P. Noel, B. S. Moore, ChemBioChem 2008, 9, 2215–2219.
- 18M. E. J. Houston, J. F. Honek, J. Chem. Soc. Chem. Commun. 1989, 761–762.
- 19
- 19aL. Misson, R. Burn, A. Vit, J. Hildesheim, M. Beliaeva, W. Blankenfeldt, F. P. Seebeck, ACS Chem. Biol. 2018, 13, 1333–1342;
- 19bA. Vit, L. E. Misson, W. Blankenfeldt, F. P. Seebeck, ChemBioChem 2015, 16, 119–125.
- 20J. G. McCoy, L. J. Bailey, Y. H. Ng, C. A. Bingman, R. Wrobel, A. P. M. Weber, B. G. Fox, G. N. Phillips, Proteins Struct. Funct. Bioinf. 2009, 74, 368–377.
- 21S. D. Braun, J. Hofmann, A. Wensing, M. S. Ullrich, H. Weingart, B. Volksch, D. Spiteller, Appl. Environ. Microbiol. 2010, 76, 2500–2508.
- 22H. Cai, D. Dumlao, J. E. Katz, S. Clarke, Biochemistry 2001, 40, 13699–13709.
- 23J. E. Katz, D. S. Dumlao, J. I. Wasserman, M. G. Lansdown, M. E. Jung, K. F. Faull, S. Clarke, Biochemistry 2004, 43, 5976–5986.
- 24C. Sommer-Kamann, A. Fries, S. Mordhorst, J. N. Andexer, M. Muller, Angew. Chem. Int. Ed. 2017, 56, 4033–4036; Angew. Chem. 2017, 129, 4091–4094.
- 25J. C. Sadler, C. H. Chung, J. E. Mosley, G. A. Burley, L. D. Humphreys, ACS Chem. Biol. 2017, 12, 374–379.
- 26D. M. Vernon, H. J. Bohnert, EMBO J. 1992, 11, 2077–2085.
- 27I. J. W. McKean, J. C. Sadler, A. Cuetos, A. Frese, L. D. Humphreys, G. Grogan, P. A. Hoskisson, G. A. Burley, Angew. Chem. Int. Ed. 2019, 58, 17583–17588; Angew. Chem. 2019, 131, 17747–17752.
- 28J. W. Schmidberger, A. B. James, R. Edwards, J. H. Naismith, D. O'Hagan, Angew. Chem. Int. Ed. 2010, 49, 3646–3648; Angew. Chem. 2010, 122, 3728–3730.
- 29I. R. Bothwell, M. Luo, Org. Lett. 2014, 16, 3056–3059.
- 30
- 30aJ. M. Lipson, M. Thomsen, B. S. Moore, R. P. Clausen, J. J. La Clair, M. D. Burkart, ChemBioChem 2013, 14, 950–953;
- 30bS. Mordhorst, A. Maurer, D. Popadic, J. Brech, J. N. Andexer, ChemCatChem 2017, 9, 4164–4168.