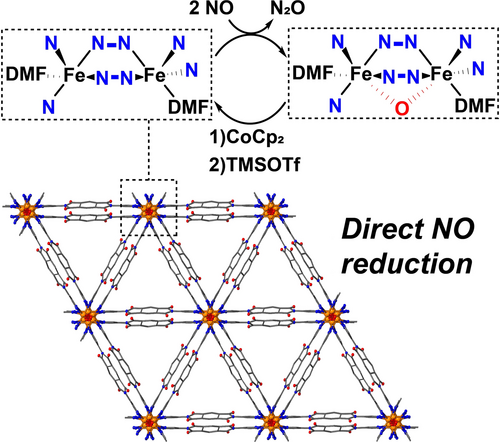
Direct NO Reduction by a Biomimetic Iron(II) Pyrazolate MOF
Dr. Zhongzheng Cai
Department of Chemistry and Biochemistry, The Ohio State University, 100 West 18th Ave, Columbus, OH, 43210 USA
Search for more papers by this authorDr. Wenjie Tao
Department of Chemistry and Biochemistry, The Ohio State University, 100 West 18th Ave, Columbus, OH, 43210 USA
Search for more papers by this authorDr. Curtis E. Moore
Department of Chemistry and Biochemistry, The Ohio State University, 100 West 18th Ave, Columbus, OH, 43210 USA
Search for more papers by this authorProf. Dr. Shiyu Zhang
Department of Chemistry and Biochemistry, The Ohio State University, 100 West 18th Ave, Columbus, OH, 43210 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Casey R. Wade
Department of Chemistry and Biochemistry, The Ohio State University, 100 West 18th Ave, Columbus, OH, 43210 USA
Search for more papers by this authorDr. Zhongzheng Cai
Department of Chemistry and Biochemistry, The Ohio State University, 100 West 18th Ave, Columbus, OH, 43210 USA
Search for more papers by this authorDr. Wenjie Tao
Department of Chemistry and Biochemistry, The Ohio State University, 100 West 18th Ave, Columbus, OH, 43210 USA
Search for more papers by this authorDr. Curtis E. Moore
Department of Chemistry and Biochemistry, The Ohio State University, 100 West 18th Ave, Columbus, OH, 43210 USA
Search for more papers by this authorProf. Dr. Shiyu Zhang
Department of Chemistry and Biochemistry, The Ohio State University, 100 West 18th Ave, Columbus, OH, 43210 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Casey R. Wade
Department of Chemistry and Biochemistry, The Ohio State University, 100 West 18th Ave, Columbus, OH, 43210 USA
Search for more papers by this authorGraphical Abstract
Abstract
A novel metal-organic framework (MOF) containing one-dimensional, Fe2+ chains bridged by dipyrazolate linkers and N,N-dimethylformamide (DMF) ligands has been synthesized. The unusual chain-type metal nodes feature accessible coordination sites on adjacent metal centers, resulting in motifs that are reminiscent of the active sites in non-heme diiron enzymes. The MOF facilitates direct reduction of nitric oxide (NO), producing nearly quantitative yields of nitrous oxide (N2O) and emulating the reactivity of flavodiiron nitric oxide reductases (FNORs). The ferrous form of the MOF can be regenerated via a synthetic cycle involving reduction with cobaltocene (CoCp2) followed by reaction with trimethylsilyl triflate (TMSOTf).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202108095-sup-0001-misc_information.pdf3.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1I. Nath, J. Chakraborty, F. Verpoort, Chem. Soc. Rev. 2016, 45, 4127–4170.
- 2X. Liu, Y. Zhou, J. Zhang, L. Tang, L. Luo, G. Zeng, ACS Appl. Mater. Interfaces 2017, 9, 20255–20275.
- 3K. Chen, C.-D. Wu, Coord. Chem. Rev. 2019, 378, 445–465.
- 4J. R. Bour, A. M. Wright, X. He, M. Dincă, Chem. Sci. 2020, 11, 1728–1737.
- 5D. Y. Osadchii, A. I. Olivos-Suarez, Á. Szécsényi, G. Li, M. A. Nasalevich, I. A. Dugulan, P. S. Crespo, E. J. M. Hensen, S. L. Veber, M. V. Fedin, G. Sankar, E. A. Pidko, J. Gascon, ACS Catal. 2018, 8, 5542–5548.
- 6Á. Szécsényi, G. Li, J. Gascon, E. A. Pidko, Chem. Sci. 2018, 9, 6765–6773.
- 7T. Nagano, T. Yoshimura, Chem. Rev. 2002, 102, 1235–1270.
- 8A. W. Carpenter, M. H. Schoenfisch, Chem. Soc. Rev. 2012, 41, 3742.
- 9D. M. Kurtz, Jr., Dalton Trans. 2007, 4115–4121.
- 10S. Khatua, A. Majumdar, J. Inorg. Biochem. 2015, 142, 145–153.
- 11T. C. Berto, A. L. Speelman, S. Zheng, N. Lehnert, Coord. Chem. Rev. 2013, 257, 244–259.
- 12N. Lehnert, K. Fujisawa, S. Camarena, H. T. Dong, C. J. White, ACS Catal. 2019, 9, 10499–10518.
- 13M. Jana, C. J. White, N. Pal, S. Demeshko, C. Cordes (née Kupper), F. Meyer, N. Lehnert, A. Majumdar, J. Am. Chem. Soc. 2020, 142, 6600–6616.
- 14H. T. Dong, C. J. White, B. Zhang, C. Krebs, N. Lehnert, J. Am. Chem. Soc. 2018, 140, 13429–13440.
- 15{FeNO}7 is the Enemark–Feltham notation for ferrous-nitrosyl. See: J. H. Enemark, R. D. Feltham, Coord. Chem. Rev. 1974, 13, 339–406.
- 16T. Hayashi, J. D. Caranto, D. A. Wampler, D. M. Kurtz, P. Moënne-Loccoz, Biochemistry 2010, 49, 7040–7049.
- 17J. D. Caranto, A. Weitz, N. Giri, M. P. Hendrich, D. M. Kurtz, Biochemistry 2014, 53, 5631–5637.
- 18J. D. Caranto, A. Weitz, M. P. Hendrich, D. M. Kurtz, J. Am. Chem. Soc. 2014, 136, 7981–7992.
- 19A. C. McKinlay, J. F. Eubank, S. Wuttke, B. Xiao, P. S. Wheatley, P. Bazin, J.-C. Lavalley, M. Daturi, A. Vimont, G. De Weireld, P. Horcajada, C. Serre, R. E. Morris, Chem. Mater. 2013, 25, 1592–1599.
- 20J. F. Eubank, P. S. Wheatley, G. Lebars, A. C. McKinlay, H. Leclerc, P. Horcajada, M. Daturi, A. Vimont, R. E. Morris, C. Serre, APL Mater. 2014, 2, 124112.
- 21E. D. Bloch, W. L. Queen, S. Chavan, P. S. Wheatley, J. M. Zadrozny, R. Morris, C. M. Brown, C. Lamberti, S. Bordiga, J. R. Long, J. Am. Chem. Soc. 2015, 137, 3466–3469.
- 22C. K. Brozek, J. T. Miller, S. A. Stoian, M. Dincă, J. Am. Chem. Soc. 2015, 137, 7495–7501.
- 23J. Jover, C. K. Brozek, M. Dincǎ, N. López, Chem. Mater. 2019, 31, 8875–8885.
- 24A. M. Wright, C. Sun, M. Dincă, J. Am. Chem. Soc. 2021, 143, 681–686.
- 25K. Sumida, S. Horike, S. S. Kaye, Z. R. Herm, W. L. Queen, C. M. Brown, F. Grandjean, G. J. Long, A. Dailly, J. R. Long, Chem. Sci. 2010, 1, 184–191.
- 26A. Jaffe, M. E. Ziebel, D. M. Halat, N. Biggins, R. A. Murphy, K. Chakarawet, J. A. Reimer, J. R. Long, J. Am. Chem. Soc. 2020, 142, 14627–14637.
- 27Deposition Number 2054298 (for 2-OH) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 28R. Silaghi-Dumitrescu, D. M. Kurtz, L. G. Ljungdahl, W. N. Lanzilotta, Biochemistry 2005, 44, 6492–6501.
- 29Z. R. Herm, B. M. Wiers, J. A. Mason, J. M. van Baten, M. R. Hudson, P. Zajdel, C. M. Brown, N. Masciocchi, R. Krishna, J. R. Long, Science 2013, 340, 960–964.
- 30N. Biggins, M. E. Ziebel, M. I. Gonzalez, J. R. Long, Chem. Sci. 2020, 11, 9173–9180.
- 31Y.-J. Qi, Y.-J. Wang, X.-X. Li, D. Zhao, Y.-Q. Sun, S.-T. Zheng, Cryst. Growth Des. 2018, 18, 7383–7390.
- 32K. I. Hadjiivanov, D. A. Panayotov, M. Y. Mihaylov, E. Z. Ivanova, K. K. Chakarova, S. M. Andonova, N. L. Drenchev, Chem. Rev. 2021, 121, 1286—1424.
- 33J. A. McCleverty, Chem. Rev. 2004, 104, 403–418.
- 34J. Li, A. Banerjee, P. L. Pawlak, W. W. Brennessel, F. A. Chavez, Inorg. Chem. 2014, 53, 5414–5416.
- 35K. D. Karlin, D. L. Lewis, H. N. Rabinowitz, S. J. Lippard, J. Am. Chem. Soc. 1974, 96, 6519–6521.
- 36S. I. Kalläne, A. W. Hahn, T. Weyhermüller, E. Bill, F. Neese, S. DeBeer, M. van Gastel, Inorg. Chem. 2019, 58, 5111–5125.
- 37A. M. Wright, T. W. Hayton, Inorg. Chem. 2015, 54, 9330–9341.
- 38K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B, Wiley, Hoboken, 2008.
- 39C. Van Stappen, N. Lehnert, Inorg. Chem. 2018, 57, 4252–4269.
- 40D. M. Kurtz, Chem. Rev. 1990, 90, 585–606.
- 41R. S. Hay-Motherwell, G. Wilkinson, T. K. N. Sweet, M. B. Hursthouse, J. Chem. Soc. Dalton Trans. 1994, 2223–2232.