Organocatalytic Enantioselective Construction of Chiral Azepine Skeleton Bearing Multiple-Stereogenic Elements
Shengli Huang
Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, Chemical Biology Research Center, School of Pharmaceutical Sciences, Chongqing University, Chongqing, 401331 P. R. China
Search for more papers by this authorHaojun Wen
Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, Chemical Biology Research Center, School of Pharmaceutical Sciences, Chongqing University, Chongqing, 401331 P. R. China
Search for more papers by this authorYuhong Tian
Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, Chemical Biology Research Center, School of Pharmaceutical Sciences, Chongqing University, Chongqing, 401331 P. R. China
Search for more papers by this authorPengfei Wang
Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, Chemical Biology Research Center, School of Pharmaceutical Sciences, Chongqing University, Chongqing, 401331 P. R. China
Search for more papers by this authorWenling Qin
Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, Chemical Biology Research Center, School of Pharmaceutical Sciences, Chongqing University, Chongqing, 401331 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hailong Yan
Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, Chemical Biology Research Center, School of Pharmaceutical Sciences, Chongqing University, Chongqing, 401331 P. R. China
Search for more papers by this authorShengli Huang
Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, Chemical Biology Research Center, School of Pharmaceutical Sciences, Chongqing University, Chongqing, 401331 P. R. China
Search for more papers by this authorHaojun Wen
Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, Chemical Biology Research Center, School of Pharmaceutical Sciences, Chongqing University, Chongqing, 401331 P. R. China
Search for more papers by this authorYuhong Tian
Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, Chemical Biology Research Center, School of Pharmaceutical Sciences, Chongqing University, Chongqing, 401331 P. R. China
Search for more papers by this authorPengfei Wang
Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, Chemical Biology Research Center, School of Pharmaceutical Sciences, Chongqing University, Chongqing, 401331 P. R. China
Search for more papers by this authorWenling Qin
Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, Chemical Biology Research Center, School of Pharmaceutical Sciences, Chongqing University, Chongqing, 401331 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hailong Yan
Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, Chemical Biology Research Center, School of Pharmaceutical Sciences, Chongqing University, Chongqing, 401331 P. R. China
Search for more papers by this authorGraphical Abstract
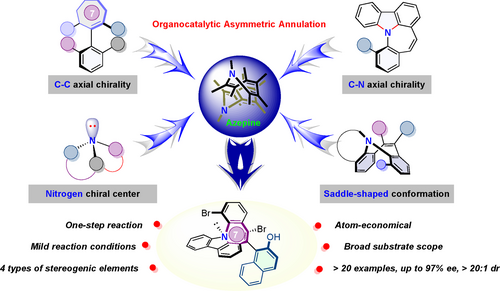
An organocatalytic enantioselective method for the preparation of polychiral molecules via vinylidene ortho-quinone methide (VQM)-mediated intramolecular electrophilic aromatic substitution was developed. With this methodology, four types of stereogenic elements including chiral nitrogen center, C−N axial chirality, C−C axial chirality and conformational behavior of the seven-membered ring were stereoselectively constructed.
Abstract
Enantioselective construction of molecules bearing multiple stereogenic elements is increasingly related to the synthesis of enantiopure natural products, pharmaceuticals, and functional materials. However, atom-economical and enantioselective approaches to install multiple stereogenic elements in a small molecular template by limited chemical transformation remain challenging. We describe an organocatalytic enantioselective method for the preparation of polychiral molecules bearing four types of stereogenic elements in fused azepines via vinylidene ortho-quinone methide (VQM)-mediated intramolecular electrophilic aromatic substitution. This method was proved robust with a wide range of substrate scope (46–92 % yield), with excellent diastereoselectivity (>20:1 dr) and enantioselectivity achieved (up to 97 % ee). Optical properties and Ru3+-induced fluorescence responses of these compounds suggest their potential applications in optoelectronic materials and heavy metal ion detection.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202108040-sup-0001-cif.zip702.5 KB | Supporting Information |
| anie202108040-sup-0001-misc_information.pdf9 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected books, see:
- 1aK. Jozwiak, W. J. Lough, I. W. Wainer, Drug Stereochemistry: Analytical Methods and Pharmacology, Informa, New York, 2012;
10.3109/9781420092394 Google Scholar
- 1bE. N. Jacobsen, A. Pfaltz, H. Yamamoto, Comprehensive Asymmetric Catalysis I-III: Suppl. I-II, Springer, Berlin, 1999;
10.1007/978-3-642-58571-5 Google Scholar
- 1cE. M. Carreira, H. Yamamoto, Comprehensive Chirality, Elsevier Science, Amsterdam, 2012.
- 2For selected reviews, see:
- 2aF. Ye, Z. Xu, L. W. Xu, Acc. Chem. Res. 2021, 54, 452–470;
- 2bG. Desimoni, G. Faita, P. Quadrelli, Chem. Rev. 2018, 118, 2080–2248;
- 2cY. Yamashita, T. Yasukawa, W. J. Yoo, T. Kitanosono, S. Kobayashi, Chem. Soc. Rev. 2018, 47, 4388–4480;
- 2dY. Wang, H. Lu, P. F. Xu, Acc. Chem. Res. 2015, 48, 1832–1844;
- 2eC. M. R. Volla, I. Atodiresei, M. Rueping, Chem. Rev. 2014, 114, 2390–2431;
- 2fH. Pellissier, Chem. Rev. 2013, 113, 442–524.
- 3For a recent review, see: X. Bao, J. Rodriguez, D. Bonne, Angew. Chem. Int. Ed. 2020, 59, 12623–12634; Angew. Chem. 2020, 132, 12723–12734.
- 4For selected examples, see:
- 4aW. Xia, Q.-J. An, S.-H. Xiang, S. Li, Y.-B. Wang, B. Tan, Angew. Chem. Int. Ed. 2020, 59, 6775–6779; Angew. Chem. 2020, 132, 6841–6845;
- 4bO. M. Beleh, E. Miller, F. D. Toste, S. J. Mille, J. Am. Chem. Soc. 2020, 142, 16461–16470;
- 4cH. Takano, N. Shiozawa, Y. Imai, K. S. Kanyiva, T. Shibata, J. Am. Chem. Soc. 2020, 142, 4714–4722;
- 4dT. Shibata, T. Fujimoto, K. Yokota, K. Takagi, J. Am. Chem. Soc. 2004, 126, 8382–8383;
- 4eY. Tan, S. Jia, F. Hu, Y. Liu, L. Peng, D. Li, H. Yan, J. Am. Chem. Soc. 2018, 140, 16893–16898;
- 4fQ. Dherbassy, J. P. Djukic, J. Wencel-Delord, F. Colobert, Angew. Chem. Int. Ed. 2018, 57, 4668–4672; Angew. Chem. 2018, 130, 4758–4762;
- 4gD. Lotter, A. Castrogiovanni, M. Neuburger, C. Sparr, ACS Cent. Sci. 2018, 4, 656–660;
- 4hK. T. Barrett, A. J. Metrano, P. R. Rablen, S. J. Miller, Nature 2014, 509, 71–75;
- 4iQ. Gao, C. Wu, S. Deng, L. Li, Z.-S. Liu, Y. Hua, J. Ye, C. Liu, H.-G. Cheng, H. Cong, Y. Jiao, Q. Zhou, J. Am. Chem. Soc. 2021, 143, 7253–7260.
- 5For selected examples, see:
- 5aA. Yubuta, T. Hosokawa, M. Gon, K. Tanaka, Y. Chujo, A. Tsurusaki, K. Kamikawa, J. Am. Chem. Soc. 2020, 142, 10025–10033;
- 5bB. Liu, M. Böckmann, W. Jiang, N. L. Doltsinis, Z. Wang, J. Am. Chem. Soc. 2020, 142, 7092–7099;
- 5cS. K. Pedersen, K. Eriksen, M. Pittelkow, Angew. Chem. Int. Ed. 2019, 58, 18419–18423; Angew. Chem. 2019, 131, 18590–18594;
- 5dK. Nakamura, S. Furumi, M. Takeuchi, T. Shibuya, K. Tanaka, J. Am. Chem. Soc. 2014, 136, 5555–5558;
- 5eS. Kinoshita, R. Yamano, Y. Shibata, Y. Tanaka, K. Hanada, T. Matsumoto, K. Miyamoto, A. Muranaka, M. Uchiyama, K. Tanaka, Angew. Chem. Int. Ed. 2020, 59, 11020–11027; Angew. Chem. 2020, 132, 11113–11120;
- 5fT. Hosokawa, Y. Takahashi, T. Matsushima, S. Watanabe, S. Kikkawa, I. Azumaya, A. Tsurusaki, K. Kamikawa, J. Am. Chem. Soc. 2017, 139, 18512–18521;
- 5gM. Satoh, Y. Shibata, K. Tanaka, Chem. Eur. J. 2018, 24, 5434–5438;
- 5hY. Kimura, Y. Shibata, K. Noguchi, K. Tanaka, Eur. J. Org. Chem. 2019, 1390–1396.
- 6G. Wu, Y. Liu, Z. Yang, T. Jiang, N. Katakam, H. Rouh, L. Ma, Y. Tang, S. Ahmed, A. U. Rahman, H. Huang, D. Unruh, G. Li, Natl. Sci. Rev. 2020, 7, 588–599.
- 7
- 7aA. Huang, Li. Zhang, D. Li, Y. Liu, H. Yan, W. Li, Org. Lett. 2019, 21, 95–99;
- 7bW. Zhang, S. Wei, W. Wang, J. Qu, B. Wang, Chem. Commun. 2021, 57, 6550–6553.
- 8
- 8aT. Böttcher, J. Chem. Inf. Model. 2016, 56, 462–470;
- 8bM. Bihani, J. C. G. Zhao, Adv. Synth. Catal. 2017, 359, 534–575;
- 8cS. Krautwald, E. M. Carreira, J. Am. Chem. Soc. 2017, 139, 5627–5639;
- 8dJ. Bruffaerts, D. Pierrot, I. Marek, Nat. Chem. 2018, 10, 1164–1170.
- 9
- 9aI. Kawashima, H. Imoto, M. Ishida, H. Furuta, S. Yamamoto, M. Mitsuishi, S. Tanaka, T. Fujii, K. N. Kawashima, Angew. Chem. Int. Ed. 2019, 58, 11686–11690; Angew. Chem. 2019, 131, 11812–11816;
- 9bL. J. Krixka, A. Ledwith, Chem. Rev. 1974, 74, 101–123;
- 9cD. Shukla, P. Wan, J. Am. Chem. Soc. 1993, 115, 2990–2991;
- 9dT. Shibata, N. Uno, T. Sasaki, H. Takano, T. Sato, K. S. Kanyiva, J. Org. Chem. 2018, 83, 3426–3432;
- 9eM. L. G. Borst, R. E. Bulo, C. W. Winkel, D. J. Gibney, A. W. Ehlers, M. Schakel, M. Lutz, A. L. Spek, K. Lammertsma, J. Am. Chem. Soc. 2005, 127, 5800–5801;
- 9fB. Quillian, Y. Wang, P. Wei, C. S. Wannere, P. v. R. Schleyer, G. H. Robinson, J. Am. Chem. Soc. 2007, 129, 13380–13381;
- 9gK. Schickedanz, J. Radtke, M. Bolte, H. W. Lerner, M. Wagner, J. Am. Chem. Soc. 2017, 139, 2842–2851.
- 10
- 10aJ. J. Vaquero, A. M. Cuadro, B. Herradn, Modern Heterocyclic Chemistry, Wiley-VCH, Weinheim, 2011;
- 10bK. Yamamoto, S. Yamazaki, Y. Kohashi, I. Murata, Tetrahedron Lett. 1982, 23, 3195–3198;
- 10cW. Tochtermann, C. Franke, Angew. Chem. Int. Ed. Engl. 1967, 6, 370; Angew. Chem. 1967, 79, 319.
- 11
- 11aY. Tahara, R. Matsubara, A. Mitake, T. Sato, K. S. Kanyiva, T. Shibata, Angew. Chem. Int. Ed. 2016, 55, 4552–4556; Angew. Chem. 2016, 128, 4628–4632;
- 11bA. Mitake, T. Fusamae, K. S. Kanyiva, T. Shibata, Eur. J. Org. Chem. 2017, 7266–7270.
- 12E. A. Stone, K. J. Cutrona, S. J. Miller, J. Am. Chem. Soc. 2020, 142, 12690–12698.
- 13For selected reviews, see:
- 13aJ. Rodriguez, D. Bonne, Chem. Commun. 2019, 55, 11168–11170;
- 13bS. Arae, M. Furusawa, S. Beppu, K. Igawa, K. Tomooka, R. Irie, Chimia 2018, 72, 892–899.
- 14For selected examples, see:
- 14aF. Doria, C. Percivalle, M. Freccero, J. Org. Chem. 2012, 77, 3615–3619;
- 14bM. Furusawa, K. Arita, T. Imahori, K. Igawa, K. Tomooka, R. Irie, Tetrahedron Lett. 2013, 54, 7107–7110;
- 14cX. Wu, L. Xue, D. Li, S. Jia, J. Ao, J. Deng, H. Yan, Angew. Chem. Int. Ed. 2017, 56, 13722–13726; Angew. Chem. 2017, 129, 13910–13914;
- 14dS. Jia, Z. Chen, N. Zhang, Y. Tan, Y. Liu, J. Deng, H. Yan, J. Am. Chem. Soc. 2018, 140, 7056–7060;
- 14eS. Arae, S. Beppu, T. Kawatsu, K. Igawa, K. Tomooka, R. Irie, Org. Lett. 2018, 20, 4796–4800;
- 14fL. Peng, D. Xu, X. Yang, J. Tang, X. Feng, S.-L. Zhang, H. Yan, Angew. Chem. Int. Ed. 2019, 58, 216–220; Angew. Chem. 2019, 131, 222–226;
- 14gS. Jia, S. Li, Y. Liu, W. Qin, H. Yan, Angew. Chem. Int. Ed. 2019, 58, 18496–18501; Angew. Chem. 2019, 131, 18667–18672;
- 14hL. Peng, K. Li, C. Xie, S. Li, D. Xu, W. Qin, H. Yan, Angew. Chem. Int. Ed. 2019, 58, 17199–17204; Angew. Chem. 2019, 131, 17359–17364;
- 14iY.-B. Wang, P. Yu, Z.-P. Zhou, J. Zhang, J. Wang, S.-H. Luo, Q.-S. Gu, K. N. Houk, B. Tan, Nat. Catal. 2019, 2, 504–513;
- 14jL. Zhang, J. Shen, S. Wu, G. Zhong, Y.-B. Wang, B. Tan, Angew. Chem. Int. Ed. 2020, 59, 23077–23082; Angew. Chem. 2020, 132, 23277–23282.
- 15For selected book and reviews on organocatalysis, see:
- 15aC. E. Song, Cinchona Alkaloids in Synthesis and Catalysis, Ligands, Immobilization and Organocatalysis, Wiley-VCH, Weinheim, 2009;
10.1002/9783527628179 Google Scholar
- 15bS.-K. Tian, Y. Chen, J. Hang, L. Tang, P. McDaid, L. Deng, Acc. Chem. Res. 2004, 37, 621–631;
- 15cA. G. Doyle, E. N. Jacobsen, Chem. Rev. 2007, 107, 5713–5743;
- 15dD. W. C. MacMillan, Nature 2008, 455, 304–308.
- 16
- 16aJ. P. Malerich, K. Hagihara, V. H. Rawal, J. Am. Chem. Soc. 2008, 130, 14416–14417;
- 16bP. Chauhan, S. Mahajan, U. Kaya, D. Hack, D. Enders, Adv. Synth. Catal. 2015, 357, 253–281.
- 17
- 17aM. Gómez-Gallego, M. A. Sierra, Chem. Rev. 2011, 111, 4857–4963;
- 17bS. S. Glad, F. Jensen, J. Org. Chem. 1997, 62, 253–260;
- 17cL. Do Amaral, H. G. Bull, E. H. Cordes, J. Am. Chem. Soc. 1972, 94, 7579–7580;
- 17dA. Streitwieser, Jr., R. H. Jagow, R. C. Fahey, S. Suzuki, J. Am. Chem. Soc. 1958, 80, 2326–2332.
- 18
- 18aP. B. D. de la Mare, T. M. Dunn, J. T. Harvey, J. Chem. Soc. 1957, 918–928;
- 18bD. B. Denney, P. P. Klemchuk, J. Am. Chem. Soc. 1958, 80, 3289–3290;
- 18cI. Szele, Helv. Chim. Acta 1981, 64, 2733;
- 18dP. de Vaal, G. Lodder, J. Cornelisse, Tetrahedron 1986, 42, 4585–4590;
- 18eC. Sandford, L. R. Fries, T. E. Ball, S. D. Minteer, M. S. Sigman, J. Am. Chem. Soc. 2019, 141, 18877–18889.
- 19Deposition Numbers 2080614 (for 2b), 2080617 (for 2f), 2080618 (for 2g), 2092561 (for 4a-major), and 2080619 (for 4b-minor) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.