Asymmetric Alkylation of Ketones Catalyzed by Engineered TrpB
Graphical Abstract
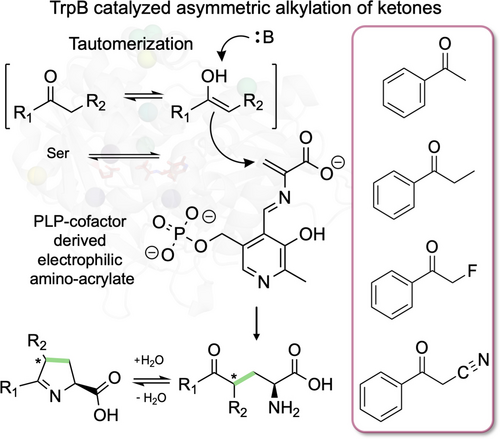
Ketone-derived enolates can serve as nucleophiles for tryptophan synthase β-subunit (TrpB) catalysed reactions. The transient nucleophilic enolate species is intercepted by TrpB, which facilitates selective C−C bond formation, resulting in the asymmetric alkylation of ketones such as propiophenone and 2-fluoroacetophenone. The products spontaneously cyclize to form highly substituted nitrogen heterocycles that can be reduced to form proline analogs.
Abstract
The β-subunit of tryptophan synthase (TrpB) catalyzes a PLP-mediated β-substitution reaction between indole and serine to form L-Trp. A succession of TrpB protein engineering campaigns to expand the enzyme's nucleophile substrate range has enabled the biocatalytic production of diverse non-canonical amino acids (ncAAs). Here, we show that ketone-derived enolates can serve as nucleophiles in the TrpB reaction to achieve the asymmetric alkylation of ketones, an outstanding challenge in synthetic chemistry. We engineered TrpB by directed evolution to catalyze the asymmetric alkylation of propiophenone and 2-fluoroacetophenone with a high degree of selectivity. In reactions with propiophenone, preference for the opposite product diastereomer emerges over the course of evolution, demonstrating that full control over the stereochemistry at the new chiral center can be achieved. The addition of this new reaction to the TrpB platform is a crucial first step toward the development of efficient methods to synthesize non-canonical prolines and other chirally dense nitrogen heterocycles.
Conflict of interest
The authors declare no conflict of interest.