The Role of Organoferrates in Iron-Catalyzed Cross-Couplings
M. Sc. Sebastian Sandl
Department of Chemistry, University of Hamburg, Martin Luther King Platz 6, 20146 Hamburg, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Axel Jacobi von Wangelin
Department of Chemistry, University of Hamburg, Martin Luther King Platz 6, 20146 Hamburg, Germany
Search for more papers by this authorM. Sc. Sebastian Sandl
Department of Chemistry, University of Hamburg, Martin Luther King Platz 6, 20146 Hamburg, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Axel Jacobi von Wangelin
Department of Chemistry, University of Hamburg, Martin Luther King Platz 6, 20146 Hamburg, Germany
Search for more papers by this authorDedicated to Professor Klaus Jonas
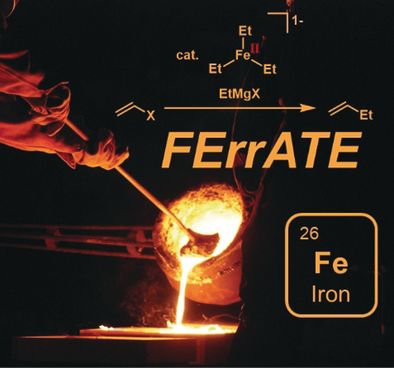
Graphical Abstract
Recent groundbreaking studies on organoferrates have demonstrated that coordinatively unsaturated three-coordinate-σ-alkylferrates are active catalysts in Fe-catalyzed cross-couplings with Grignard reagents and that pronounced solvent and counterion effects dictate metalate speciation and catalyst activity. Thanks to modern spectroscopic methods, sensitive catalyst intermediates could be analyzed.
References
- 1
- 1aA. Fürstner, A. Leitner, Angew. Chem. Int. Ed. 2002, 41, 609–612; Angew. Chem. 2002, 114, 632–635;
- 1bA related NMP-free procedure afforded good yields in the cross-coupling with 4-chlorobenzoate; NMP was further shown to enhance the cross-coupling yields of more challenging substrates: E. Bisz, M. Szostak, Green Chem. 2017, 19, 5361–5366.
- 2
- 2aW. M. Czaplik, M. Mayer, J. Cvengroš, A. Jacobi von Wangelin, ChemSusChem 2009, 2, 396–417;
- 2bI. Bauer, H. Knölker, Chem. Rev. 2015, 115, 3170–3387;
- 2cR. B. Bedford, P. B. Brenner, Top. Organomet. Chem. 2015, 50, 19–46;
- 2dC. Cassani, G. Bergonzini, C.-J. Wallentin, ACS Catal. 2016, 6, 1640–1648;
- 2eT. L. Mako, J. A. Byers, Inorg. Chem. Front. 2016, 3, 766–790;
- 2fA. Piontek, E. Bisz, M. Szostak, Angew. Chem. Int. Ed. 2018, 57, 11116–11128; Angew. Chem. 2018, 130, 11284–11297.
- 3C. E. I. Knappke, A. Jacobi von Wangelin, Chem. Soc. Rev. 2011, 40, 4948–4962.
- 4J. A. Wanklyn, Liebigs Ann. Chem. 1858, 107, 125–128.
10.1002/jlac.18581070119 Google Scholar
- 5
- 5aJ. P. Collman, Acc. Chem. Res. 1975, 8, 342–347;
- 5bF. Mongin, A. Harrison-Marchand, Chem. Rev. 2013, 113, 7563–7727;
- 5cA. Gómez-Suárez, D. J. Nelson, S. P. Nolan, Adv. Organomet. Chem. 2018, 69, 283–327.
- 6
- 6aR. E. Mulvey, F. Mongin, M. Uchiyama, Y. Kondo, Angew. Chem. Int. Ed. 2007, 46, 3802–3824; Angew. Chem. 2007, 119, 3876–3899;
- 6bS. D. Robertson, M. Uzelac, R. E. Mulvey, Chem. Rev. 2019, 119, 8332–8405.
- 7
- 7aK. Jonas, L. Schieferstein, C. Krüger, Y.-H. Tsay, Angew. Chem. Int. Ed. Engl. 1979, 18, 550–551; Angew. Chem. 1979, 91, 590–591;
- 7bK. Jonas, Angew. Chem. Int. Ed. Engl. 1985, 24, 295–311; Angew. Chem. 1985, 97, 292–307;
- 7cThe field of main-group organometallic and organometalate reagents has recently been significantly expanded by new procedures, new reagents, and new applications to organic synthesis, with a major focus on cross-coupling reactions: Handbook of Functionalized Organometallics: Applications in Synthesis (Ed.: ), Wiley-VCH, Weinheim, 2005.
- 8
- 8aW. W. Brennessel, V. G. Young, Jr., J. E. Ellis, Angew. Chem. Int. Ed. 2002, 41, 1211–1215;
10.1002/1521-3773(20020402)41:7<1211::AID-ANIE1211>3.0.CO;2-I CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 1259–1263;
- 8bJ. E. Ellis, Inorg. Chem. 2006, 45, 3167–3186;
- 8cJ. E. Ellis, Dalton Trans. 2019, 48, 9538–9563.
- 9
- 9aH. J. Spiegl, G. Groh, H. J. Berthold, Z. Anorg. Allg. Chem. 1973, 398, 225–230;
- 9bA. Fürstner, H. Krause, C. W. Lehmann, Angew. Chem. Int. Ed. 2006, 45, 440–444; Angew. Chem. 2006, 118, 454–458;
- 9cA. Fürstner, R. Martin, H. Krause, G. Seidel, R. Goddard, C. W. Lehmann, J. Am. Chem. Soc. 2008, 130, 8773–8787.
- 10T. Kauffmann, Angew. Chem. Int. Ed. Engl. 1996, 35, 386–403; Angew. Chem. 1996, 108, 401–418.
- 11
- 11aW. Seidel, K.-J. Lattermann, Z. Anorg. Allg. Chem. 1982, 488, 69–74;
- 11bR. B. Bedford, P. B. Brenner, E. Carter, P. M. Cogswell, M. F. Haddow, J. N. Harvey, D. M. Murphy, J. Nunn, C. H. Woodall, Angew. Chem. Int. Ed. 2014, 53, 1804–1808; Angew. Chem. 2014, 126, 1835–1839.
- 12R. B. Bedford, Acc. Chem. Res. 2015, 48, 1485–1493.
- 13S. B. Muñoz, S. L. Daifuku, J. D. Sears, T. M. Baker, S. H. Carpenter, W. W. Brennessel, M. L. Neidig, Angew. Chem. Int. Ed. 2018, 57, 6496–6500; Angew. Chem. 2018, 130, 6606–6610.
- 14J. D. Sears, S. B. Muñoz, S. L. Daifuku, A. A. Shaps, S. H. Carpenter, W. W. Brennessel, M. L. Neidig, Angew. Chem. Int. Ed. 2019, 58, 2769–2773; Angew. Chem. 2019, 131, 2795–2799.
- 15S. B. Muñoz, S. L. Daifuku, W. W. Brennessel, M. L. Neidig, J. Am. Chem. Soc. 2016, 138, 7492–7495.
- 16T. Parchomyk, S. Demeshko, F. Meyer, K. Koszinowski, J. Am. Chem. Soc. 2018, 140, 9709–9720.
- 17R. S. Smith, J. K. Kochi, J. Org. Chem. 1976, 41, 502–509.
- 18G. Cahiez, H. Avedissian, Synthesis 1998, 1199–1205.
- 19M. L. Neidig, S. H. Carpenter, D. J. Curran, J. C. DeMuth, V. E. Fleischauer, T. E. Iannuzzi, P. G. N. Neate, J. D. Sears, N. J. Wolford, Acc. Chem. Res. 2019, 52, 140–150.
- 20F. Freitag, T. Irrgang, R. Kempe, J. Am. Chem. Soc. 2019, 141, 11677–11685.
- 21S. Sandl, T. M. Maier, N. P. van Leest, S. Kröncke, U. Chakraborty, S. Demeshko, K. Koszinowski, B. de Bruin, F. Meyer, M. Bodensteiner, C. Herrmann, R. Wolf, A. Jacobi von Wangelin, ACS Catal. 2019, 9, 7596–7606.
- 22
- 22aC. R. Kennedy, S. Lin, E. N. Jacobsen, Angew. Chem. Int. Ed. 2016, 55, 12596–12624; Angew. Chem. 2016, 128, 12784–12814;
- 22bS. Yamada, Chem. Rev. 2018, 118, 11353–11432;
- 22cK. T. Mahmudov, A. V. Gurbanov, F. I. Guseinov, M. F. C. Guedes da Silva, Coord. Chem. Rev. 2019, 387, 32–46.
- 23
- 23aK. Grubel, W. W. Brennessel, B. Q. Mercado, P. L. Holland, J. Am. Chem. Soc. 2014, 136, 16807–16816;
- 23bG. P. Connor, P. L. Holland, Catal. Today 2017, 286, 21–40.