Total Synthesis of (−)-Perezoperezone through an Intermolecular [5+2] Homodimerization of Hydroxy p-Quinone
Yang Long
Department of Medicinal Natural Products, West China School of Pharmacy, Sichuan University, Chengdu, 610041 P. R. China
State Key Laboratory of Phytochemistry and Plant Resources in West China, Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, China
These authors contributed equally to this work.
Search for more papers by this authorYiming Ding
State Key Laboratory of Phytochemistry and Plant Resources in West China, Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, China
University of Chinese Academy of Sciences, Beijing, 100049 China
These authors contributed equally to this work.
Search for more papers by this authorHai Wu
State Key Laboratory of Phytochemistry and Plant Resources in West China, Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorChunlei Qu
State Key Laboratory of Phytochemistry and Plant Resources in West China, Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorHong Liang
State Key Laboratory of Phytochemistry and Plant Resources in West China, Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorMin Zhang
State Key Laboratory of Phytochemistry and Plant Resources in West China, Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorXiaoli Zhao
State Key Laboratory of Phytochemistry and Plant Resources in West China, Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorXianwen Long
State Key Laboratory of Phytochemistry and Plant Resources in West China, Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorDr. Shu Wang
Department of Medicinal Natural Products, West China School of Pharmacy, Sichuan University, Chengdu, 610041 P. R. China
Search for more papers by this authorDr. Pema-Tenzin Puno
State Key Laboratory of Phytochemistry and Plant Resources in West China, Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, China
Search for more papers by this authorCorresponding Author
Dr. Jun Deng
State Key Laboratory of Phytochemistry and Plant Resources in West China, Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, China
Search for more papers by this authorYang Long
Department of Medicinal Natural Products, West China School of Pharmacy, Sichuan University, Chengdu, 610041 P. R. China
State Key Laboratory of Phytochemistry and Plant Resources in West China, Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, China
These authors contributed equally to this work.
Search for more papers by this authorYiming Ding
State Key Laboratory of Phytochemistry and Plant Resources in West China, Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, China
University of Chinese Academy of Sciences, Beijing, 100049 China
These authors contributed equally to this work.
Search for more papers by this authorHai Wu
State Key Laboratory of Phytochemistry and Plant Resources in West China, Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorChunlei Qu
State Key Laboratory of Phytochemistry and Plant Resources in West China, Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorHong Liang
State Key Laboratory of Phytochemistry and Plant Resources in West China, Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorMin Zhang
State Key Laboratory of Phytochemistry and Plant Resources in West China, Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorXiaoli Zhao
State Key Laboratory of Phytochemistry and Plant Resources in West China, Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorXianwen Long
State Key Laboratory of Phytochemistry and Plant Resources in West China, Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorDr. Shu Wang
Department of Medicinal Natural Products, West China School of Pharmacy, Sichuan University, Chengdu, 610041 P. R. China
Search for more papers by this authorDr. Pema-Tenzin Puno
State Key Laboratory of Phytochemistry and Plant Resources in West China, Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, China
Search for more papers by this authorCorresponding Author
Dr. Jun Deng
State Key Laboratory of Phytochemistry and Plant Resources in West China, Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, China
Search for more papers by this authorDedicated to Professor Handong Sun on the occasion of his 80th birthday
Graphical Abstract
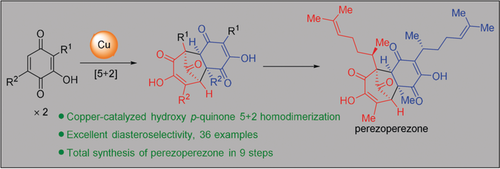
In nine steps: The first copper-catalyzed intermolecular [5+2] homodimerization of hydroxy p-quinone is presented, furnishing bicyclo[3.2.1]octadienone core structures in typically good yields and excellent diastereoselectivities. Applying this synthetic approach enables a concise nine-step total synthesis of (−)-perezoperezone from commercially available 3,5-dimethoxytoluene.
Abstract
The first copper-catalyzed intermolecular [5+2] homodimerization of hydroxy p-quinone is presented, furnishing bicyclo[3.2.1]octadienone core structures in typically good yields and excellent diastereoselectivities. Applying this synthetic approach enables a concise nine-step total synthesis of (−)-perezoperezone from commercially available 3,5-dimethoxytoluene.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201911978-sup-0001-misc_information.pdf12.7 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see:
- 1aM. Presset, Y. Coquerel, J. Rodriguez, Chem. Rev. 2013, 113, 525–595;
- 1bK. E. O. Ylijoki, J. M. Stryker, Chem. Rev. 2013, 113, 2244–2266;
- 1cS. Kamo, K. Kuramochi, K. Tsubaki, Tetrahedron Lett. 2018, 59, 224–230.
- 2For recent reviews, see:
- 2aX. Liu, Y. Hu, J. Fan, J. Zhao, S. Li, C. Li, Org. Chem. Front. 2018, 5, 1217–1228;
- 2bH. Pellissier, Adv. Synth. Catal. 2018, 360, 1551–1583;
- 2cY. Wang, Z. Yu, Acc. Chem. Res. 2015, 48, 2288–2296;
- 2dL. Jiao, Z. Yu, J. Org. Chem. 2013, 78, 6842–6848; For selected research work published recently, see:
- 2eJ. Liu, J. Wu, J. Yan, G. Mei, C. Li, J. Am. Chem. Soc. 2018, 140, 5365–5369;
- 2fC. He, J. Hu, Y. Wu, H. Ding, J. Am. Chem. Soc. 2017, 139, 6098–6101;
- 2gC. He, Z. Bai, J. Hu, B. Wang, H. Xie, L. Yu, H. Ding, Chem. Commun. 2017, 53, 8435–8438;
- 2hX. Liu, J. Liu, J. Zhao, S. Li, C. Li, Org. Lett. 2017, 19, 2742–2745;
- 2iS. M. Wilkerson-Hill, S. Sawano, R. Sarpong, J. Org. Chem. 2016, 81, 11132–11144;
- 2jC. Hu, R. Song, M. Hu, Y. Yang, J. Li, S. Luo, Angew. Chem. Int. Ed. 2016, 55, 10423–10426; Angew. Chem. 2016, 128, 10579–10582;
- 2kM. P. D'Erasmo, C. Meck, C. A. Lewis, R. P. Murelli, J. Org. Chem. 2016, 81, 3744–3751;
- 2lJ. Feng, T. Lin, H. Wu, J. Zhang, Angew. Chem. Int. Ed. 2015, 54, 15854–15858; Angew. Chem. 2015, 127, 16080–16084;
- 2mJ. Feng, T. Lin, H. Wu, J. Zhang, J. Am. Chem. Soc. 2015, 137, 3787–3790;
- 2nG. Mei, X. Liu, C. Qiao, W. Chen, C. Li, Angew. Chem. Int. Ed. 2015, 54, 1754–1758; Angew. Chem. 2015, 127, 1774–1778;
- 2oG. Mei, H. Yuan, Y. Gu, W. Chen, L. Chung, C. Li, Angew. Chem. Int. Ed. 2014, 53, 11051–11055; Angew. Chem. 2014, 126, 11231–11235;
- 2pX. Hong, M. C. Stevens, P. Liu, P. A. Wender, K. N. Houk, J. Am. Chem. Soc. 2014, 136, 17273–17283;
- 2qM. R. Witten, E. N. Jacobsen, Angew. Chem. Int. Ed. 2014, 53, 5912–5916; Angew. Chem. 2014, 126, 6022–6026.
- 3One example is the formation of purpurogallin: W. Dürckheimer, E. F. Paulus, Angew. Chem. Int. Ed. Engl. 1985, 24, 123–135; Angew. Chem. 1985, 97, 125–127.
- 4I. H. Sánchez, R. Yáńez, R. Enríquez, P. Joseph-Nathan, J. Org. Chem. 1981, 46, 2818–2819.
- 5
- 5aN. Waizumi, A. R. Stankovic, V. H. Rawal, J. Am. Chem. Soc. 2003, 125, 13022–13023;
- 5bA. I. Kim, S. D. Rychnovsky, Angew. Chem. Int. Ed. 2003, 42, 1267–1270; Angew. Chem. 2003, 115, 1305–1308;
- 5cD. C. Harrowven, D. D. Pascoe, D. Demurtas, H. O. Bourner, Angew. Chem. Int. Ed. 2005, 44, 1221–1222; Angew. Chem. 2005, 117, 1247–1248;
- 5dA. A. Boezio, E. R. Jarvo, B. M. Lawrence, E. N. Jacobsen, Angew. Chem. Int. Ed. 2005, 44, 6046–6050; Angew. Chem. 2005, 117, 6200–6204;
- 5eH. M. L. Davies, X. Dai, M. S. Long, J. Am. Chem. Soc. 2006, 128, 2485–2490;
- 5fJ. Preindl, C. Leitner, S. Baldauf, J. Mulzer, Org. Lett. 2014, 16, 4276–4279.
- 6P. Georgantea, E. Ioannou, C. Vagias, V. Roussis, Tetrahedron Lett. 2013, 54, 6920–6922.
- 7G. Yin, Y. Wu, C. Han, X. Wang, H. Gao, Y. Yin, L. Kong, M. Yang, Org. Chem. Front. 2018, 5, 2432–2436.
- 8W. H. Lin, J. M. Fang, Y. S. Cheng, Phyochemistry 1997, 46, 169–173.
- 9
- 9aX. Long, Y. Ding, J. Deng, Angew. Chem. Int. Ed. 2018, 57, 14221–14224; Angew. Chem. 2018, 130, 14417–14420;
- 9bR. Bao, C. Tian, H. Zhang, Z. Wang, Z. Dong, Y. Li, M. Gao, H. Zhang, G. Liu, Y. Tang, Angew. Chem. Int. Ed. 2018, 57, 14216–14220; Angew. Chem. 2018, 130, 14412–14416;
- 9cJ. R. Reyes, N. Winter, L. Spessert, D. Trauner, Angew. Chem. Int. Ed. 2018, 57, 15587–15591; Angew. Chem. 2018, 130, 15813–15817.
- 10H. Yang, J. Feng, Y. Li, Y. Tang, Org. Lett. 2015, 17, 1441–1444.
- 11
- 11aD. B. Ramachary, M. A. Pasha, G. Thirupathi, Angew. Chem. Int. Ed. 2017, 56, 12930–12934; Angew. Chem. 2017, 129, 13110–13114.
- 12
- 12aY. Ando, S. Hori, T. Fukazawa, K. Ohmori, K. Suzuki, Angew. Chem. Int. Ed. 2015, 54, 9650–9653; Angew. Chem. 2015, 127, 9786–9789.
- 13
- 13aP. Ellerbrock, N. Armanino, M. K. Ilg, R. Webster, D. Trauner, Nat. Chem. 2015, 7, 879–882;
- 13bA. J. E. Novak, C. E. Grigglestone, D. Trauner, J. Am. Chem. Soc. 2019, 141, 15515–15518.
- 14
- 14aA. G. Kravina, E. M. Carreira, Angew. Chem. Int. Ed. 2018, 57, 13159–13162; Angew. Chem. 2018, 130, 13343–13346.
- 15Iron catalyzed intramolecular perezone-type [5+2] cycloaddition:
- 15aY. Liu, X. Wang, S. Chen, S. Fu, B. Liu, Org. Lett. 2018, 20, 2934–2938; Iron catalyzed oxidative phenolic coupling:
- 15bS. Gao, X. Hu, Org. Chem. Front. 2017, 4, 1493–1498.
- 16For recent review articles on Copper catalysis, see:
- 16aA. Alexakis, J. E. Bäckvall, N. Krause, O. Pàmies, M. Diéguez, Chem. Rev. 2008, 108, 2796–2823;
- 16bH. Guo, J. Ma, Angew. Chem. Int. Ed. 2006, 45, 354–366; Angew. Chem. 2006, 118, 362–375; For selected research work published recently, see:
- 16cN. R. Cichowicz, W. Kaplan, Y. Khomutnyk, B. Bhattarai, Z. Sun, P. Nagorny, J. Am. Chem. Soc. 2015, 137, 14341–14348.
- 17M. Buccini, K. A. Punch, B. Kaskow, G. R. Flematti, B. W. Skelton, L. J. Abraham, M. J. Piggott, Org. Biomol. Chem. 2014, 12, 1100–1113.
- 18
- 18aD. M. Pinkerton, M. G. Banwell, A. C. Willis, Aust. J. Chem. 2009, 62, 1639–1645;
- 18bX. Ma, J. C. Jury, M. G. Banwell, Tetrahedron Lett. 2011, 52, 2191–2194.
- 19For recent examples of Suzuki–Miyaura coupling with sterically encumbered substrates, see:
- 19aO. Navarro, R. A. Kelly, S. P. Nolan, J. Am. Chem. Soc. 2003, 125, 16194–16195;
- 19bC. Li, T. Chen, B. Li, G. Xiao, W. Tang, Angew. Chem. Int. Ed. 2015, 54, 3792–3796; Angew. Chem. 2015, 127, 3863–3867;
- 19cC. Li, G. Xiao, Q. Zhao, H. Liu, T. Wang, W. Tang, Org. Chem. Front. 2014, 1, 225–229;
- 19dG. Wu, Y. Zhang, D. Tan, F. Han, Nat. Commun. 2018, 9, 2148.
- 20
- 20aS. Yang, S. Zhu, N. Guo, S. Song, Q. Zhou, Org. Biomol. Chem. 2014, 12, 2049–2052;
- 20bZ. Wang, F. Ai, Z. Wang, W. Zhao, G. Zhu, Z. Lin, J. Sun, J. Am. Chem. Soc. 2015, 137, 383–389;
- 20cJ. Zheng, C. Margarita, S. Krajangsri, P. G. Andersson, Org. Lett. 2018, 20, 5676–5679;
- 20dZ. Zhang, J. Wang, J. Li, F. Yang, G. Liu, W. Tang, W. He, J. Fu, Y. Shen, A. Li, W. Zhang, J. Am. Chem. Soc. 2017, 139, 5558–5567.
- 21The Optical rotation of (−)-perezoperezone was disclosed by Cuevas and co-workers, see: G. Roura-Pérez, B. Quiróz, M. Aguilar-Martínez, C. Frontana, A. Solano, I. González, J. A. Bautista-Martínez, J. Jiménez-Barbero, G. Cuevas, J. Org. Chem. 2007, 72, 1883–1894.
- 22CCDC 1949322 (8 ae), 1949323 (8 ba), 1949324 (8 ca), 1949319 (8 dc), and 1949321 (8 ff) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.