Cobalt-Catalyzed Asymmetric Hydrogenation of C=N Bonds Enabled by Assisted Coordination and Nonbonding Interactions
Yanhua Hu
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorDr. Zhenfeng Zhang
School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorJian Zhang
School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorProf. Dr. Yangang Liu
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorProf. Dr. Ilya D. Gridnev
Department of Chemistry, Graduate School of Science, Tohoku University, Aramaki 3–6, Aoba-ku, Sendai, 980-8578 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Wanbin Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorYanhua Hu
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorDr. Zhenfeng Zhang
School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorJian Zhang
School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorProf. Dr. Yangang Liu
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorProf. Dr. Ilya D. Gridnev
Department of Chemistry, Graduate School of Science, Tohoku University, Aramaki 3–6, Aoba-ku, Sendai, 980-8578 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Wanbin Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorGraphical Abstract
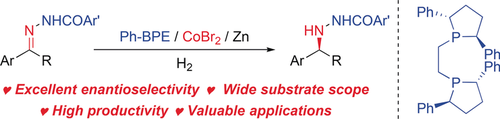
Interaction skills: Chiral nitrogen-containing compounds have been synthesized with excellent enantioselectivity (95–98 % ee) and high productivity (up to 2000 TON) by the Co-catalyzed asymmetric hydrogenation of C=N bonds. The reaction is facilitated by coordination of an NHBz group in the substrates to the cobalt atom and a nonbonding interaction with the ligand.
Abstract
An efficient cobalt-catalyzed asymmetric hydrogenation of C=N bonds has been realized. Chiral hydrazines were obtained in high yields and with excellent enantioselectivities (95–98 % ee). The hydrogenation went smoothly at up to 2000 substrate/catalyst and on a gram scale. The success of this reaction relies on the presence of an NHBz group in the substrates, with the reactivity and enantioselectivity improved by an assisted coordination to the cobalt atom and a nonbonding interaction with the ligand. Furthermore, this reaction has practical applications for the synthesis of several useful chiral nitrogen-containing compounds.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201909928-sup-0001-misc_information.pdf7.8 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. K. Friestad, Eur. J. Org. Chem. 2005, 3157–3172;
- 1bG.-Q. Lin, M.-H. Xu, Y.-W. Zhong, X.-W. Sun, Acc. Chem. Res. 2008, 41, 831–840;
- 1cC. K. Savile, J. M. Janey, E. C. Mundorff, J. C. Moore, S. Tam, W. R. Jarvis, J. C. Colbeck, A. Krebber, F. J. Fleitz, J. Brands, P. N. Devine, G. W. Huisman, G. J. Hughes, Science 2010, 329, 305–309;
- 1dY. Yang, S.-L. Shi, D. Niu, P. Liu, S. L. Buchwald, Science 2015, 349, 62–66;
- 1eM. D. Patil, G. Grogan, A. Bommarius, H. Yun, ACS Catal. 2018, 8, 10985–11015.
- 2Reviews:
- 2aJ.-H. Xie, S.-F. Zhu, Q.-L. Zhou, Chem. Rev. 2011, 111, 1713–1760;
- 2bD.-S. Wang, Q.-A. Chen, S.-M. Lu, Y.-G. Zhou, Chem. Rev. 2012, 112, 2557–2590;
- 2cQ.-A. Chen, Z.-S. Ye, Y. Duan, Y.-G. Zhou, Chem. Soc. Rev. 2013, 42, 497–511;
- 2dY. Wang, Z. Zhang, W. Zhang, Chin. J. Org. Chem. 2015, 35, 528–538;
- 2eZ. Zhang, N. A. Butt, W. Zhang, Chem. Rev. 2016, 116, 14769–14827; recent reports:
- 2fG.-S. Feng, M.-W. Chen, L. Shi, Y.-G. Zhou, Angew. Chem. Int. Ed. 2018, 57, 5853–5857; Angew. Chem. 2018, 130, 5955–5959;
- 2gY. Lou, Y. Hu, J. Lu, F. Guan, G. Gong, Q. Yin, X. Zhang, Angew. Chem. Int. Ed. 2018, 57, 14193–14197; Angew. Chem. 2018, 130, 14389–14393;
- 2hY. Chen, Y.-M. He, S. Zhang, T. Miao, Q.-H. Fan, Angew. Chem. Int. Ed. 2019, 58, 3809–3813; Angew. Chem. 2019, 131, 3849–3853;
- 2iC. Li, F. Wan, Y. Chen, H. Peng, W. Tang, S. Yu, J. C. McWilliams, J. Mustakis, L. Samp, R. J. Maguire, Angew. Chem. Int. Ed. 2019, 58, 13573–13583; Angew. Chem. 2019, 131, 13707–13717.
- 3Ti-catalyzed AH of C=N bonds:
- 3aC. A. Willoughby, S. L. Buchwald, J. Am. Chem. Soc. 1992, 114, 7562–7564;
- 3bC. A. Willoughby, S. L. Buchwald, J. Am. Chem. Soc. 1994, 116, 8952–8965;
- 3cC. A. Willoughby, S. L. Buchwald, J. Am. Chem. Soc. 1994, 116, 11703–11714;
- 3dM. Ringwald, R. Stürmer, H. H. Brintzinger, J. Am. Chem. Soc. 1999, 121, 1524–1527; Ti-catalyzed AH of C=C bonds:
- 3eR. L. Halterman, K. P. C. Vollhardt, M. E. Welker, D. Bläser, R. Boese, J. Am. Chem. Soc. 1987, 109, 8105–8107;
- 3fR. D. Broene, S. L. Buchwald, J. Am. Chem. Soc. 1993, 115, 12569–12570;
- 3gN. E. Lee, S. L. Buchwald, J. Am. Chem. Soc. 1994, 116, 5985–5986.
- 4Mn-catalyzed AH of C=O bonds:
- 4aM. B. Widegren, G. J. Harkness, A. M. Z. Slawin, D. B. Cordes, M. L. Clarke, Angew. Chem. Int. Ed. 2017, 56, 5825–5828; Angew. Chem. 2017, 129, 5919–5922;
- 4bM. Garbe, K. Junge, S. Walker, Z. Wei, H. Jiao, A. Spannenberg, S. Bachmann, M. Scalone, M. Beller, Angew. Chem. Int. Ed. 2017, 56, 11237–11241; Angew. Chem. 2017, 129, 11389–11393;
- 4cL. Zhang, Y. Tang, Z. Han, K. Ding, Angew. Chem. Int. Ed. 2019, 58, 4973–4977; Angew. Chem. 2019, 131, 5027–5031;
- 4dF. Ling, H. Hou, J. Chen, S. Nian, X. Yi, Z. Wang, D. Song, W. Zhong, Org. Lett. 2019, 21, 3937–3941.
- 5Fe-catalyzed AH of C=N bonds:
- 5aS. Zhou, S. Fleischer, K. Junge, M. Beller, Angew. Chem. Int. Ed. 2011, 50, 5120–5124; Angew. Chem. 2011, 123, 5226–5230;
- 5bP. O. Lagaditis, P. E. Sues, J. F. Sonnenberg, K. Y. Wan, A. J. Lough, R. H. Morris, J. Am. Chem. Soc. 2014, 136, 1367–1380; Fe-catalyzed AH of C=O bonds:
- 5cC. Sui-Seng, F. Freutel, A. J. Lough, R. H. Morris, Angew. Chem. Int. Ed. 2008, 47, 940–943; Angew. Chem. 2008, 120, 954–957;
- 5dY. Li, S. Yu, X. Wu, J. Xiao, W. Shen, Z. Dong, J. Gao, J. Am. Chem. Soc. 2014, 136, 4031–4039;
- 5eJ. F. Sonnenberg, K. Y. Wan, P. E. Sues, R. H. Morris, ACS Catal. 2017, 7, 316–326.
- 6Co-catalyzed AH of C=C bonds:
- 6aS. Monfette, Z. R. Turner, S. P. Semproni, P. J. Chirik, J. Am. Chem. Soc. 2012, 134, 4561–4564;
- 6bM. R. Friedfeld, M. Shevlin, J. M. Hoyt, S. W. Krska, M. T. Tudge, P. J. Chirik, Science 2013, 342, 1076–1080;
- 6cM. R. Friedfeld, M. Shevlin, G. W. Margulieux, L.-C. Campeau, P. J. Chirik, J. Am. Chem. Soc. 2016, 138, 3314–3324;
- 6dJ. Chen, C. Chen, C. Ji, Z. Lu, Org. Lett. 2016, 18, 1594–1597;
- 6eJ. Guo, X. Shen, Z. Lu, Angew. Chem. Int. Ed. 2017, 56, 615–618; Angew. Chem. 2017, 129, 630–633;
- 6fJ. Guo, B. Cheng, X. Shen, Z. Lu, J. Am. Chem. Soc. 2017, 139, 15316–15319;
- 6gM. R. Friedfeld, H. Zhong, R. T. Ruck, M. Shevlin, P. J. Chirik, Science 2018, 360, 888–893;
- 6hH. Zhong, M. R. Friedfeld, P. J. Chirik, Angew. Chem. Int. Ed. 2019, 58, 9194–9198; Angew. Chem. 2019, 131, 9292–9296; Co-catalyzed AH of C=O bonds:
- 6iD. Zhang, E.-Z. Zhu, Z.-W. Lin, Z.-B. Wei, Y.-Y. Li, J.-X. Gao, Asian J. Org. Chem. 2016, 5, 1323–1326.
- 7Ni-catalyzed AH of C=N bonds:
- 7aB. Li, J. Chen, Z. Zhang, I. D. Gridnev, W. Zhang, Angew. Chem. Int. Ed. 2019, 58, 7329–7334; Angew. Chem. 2019, 131, 7407–7412; Ni-catalyzed AH of C=C bonds:
- 7bM. Shevlin, M. R. Friedfeld, H. Sheng, N. A. Pierson, J. M. Hoyt, L.-C. Campeau, P. J. Chirik, J. Am. Chem. Soc. 2016, 138, 3562–3569;
- 7cW. Gao, H. Lv, T. Zhang, Y. Yang, L. W. Chung, Y.-D. Wu, X. Zhang, Chem. Sci. 2017, 8, 6419–6422;
- 7dY.-Q. Guan, Z. Han, X. Li, C. You, X. Tan, H. Lv, X. Zhang, Chem. Sci. 2019, 10, 252–256; Ni-catalyzed AH of C=N bonds:
- 7eH. Xu, P. Yang, P. Chuanprasit, H. Hirao, J. S. Zhou, Angew. Chem. Int. Ed. 2015, 54, 5112–5116; Angew. Chem. 2015, 127, 5201–5205;
- 7fP. Yang, L. H. Lim, P. Chuanprasit, H. Hirao, J. S. Zhou, Angew. Chem. Int. Ed. 2016, 55, 12083–12087; Angew. Chem. 2016, 128, 12262–12266;
- 7gP. Yang, C. Zhang, Y. Ma, C. Zhang, A. Li, B. Tang, J. S. Zhou, Angew. Chem. Int. Ed. 2017, 56, 14702–14706; Angew. Chem. 2017, 129, 14894–14898;
- 7hX. Zhao, H. Xu, X. Huang, J. S. Zhou, Angew. Chem. Int. Ed. 2019, 58, 292–296; Angew. Chem. 2019, 131, 298–302.
- 8Cu-catalyzed AH of C=O bonds:
- 8aH. Shimizu, D. Igarashi, W. Kuriyama, Y. Yusa, N. Sayo, T. Saito, Org. Lett. 2007, 9, 1655–1657;
- 8bK. Junge, B. Wendt, D. Addis, S. Zhou, S. Das, S. Fleischer, M. Beller, Chem. Eur. J. 2011, 17, 101–105;
- 8cS. W. Krabbe, M. A. Hatcher, R. K. Bowman, M. B. Mitchell, M. S. McClure, J. S. Johnson, Org. Lett. 2013, 15, 4560–4563;
- 8dO. V. Zatolochnaya, S. Rodríguez, Y. Zhang, K. S. Lao, S. Tcyrulnikov, G. Li, X.-J. Wang, B. Qu, S. Biswas, H. P. R. Mangunuru, D. Rivalti, J. D. Sieber, J.-N. Desrosiers, J. C. Leung, N. Grinberg, H. Lee, N. Haddad, N. K. Yee, J. J. Song, M. C. Kozlowski, C. H. Senanayake, Chem. Sci. 2018, 9, 4505–4510.
- 9
- 9aZ. Zhang, N. A. Butt, M. Zhou, D. Liu, W. Zhang, Chin. J. Chem. 2018, 36, 443–454; asymmetric hydrofunctionalization including hydrogenation:
- 9bJ. Chen, Z. Lu, Org. Chem. Front. 2018, 5, 260–273;
- 9cJ. Chen, J. Guo, Z. Lu, Chin. J. Chem. 2018, 36, 1075–1109.
- 10
- 10aH. Pellissier, H. Clavier, Chem. Rev. 2014, 114, 2775–2823;
- 10bW. Liu, B. Sahoo, K. Junge, M. Beller, Acc. Chem. Res. 2018, 51, 1858–1869;
- 10cK. Junge, V. Papa, M. Beller, Chem. Eur. J. 2019, 25, 122–143;
- 10dW. Ai, R. Zhong, X. Liu, Q. Liu, Chem. Rev. 2019, 119, 2876–2953.
- 11
- 11aY. Liu, W. Zhang, Angew. Chem. Int. Ed. 2013, 52, 2203–2206; Angew. Chem. 2013, 125, 2259–2262;
- 11bY. Liu, I. D. Gridnev, W. Zhang, Angew. Chem. Int. Ed. 2014, 53, 1901–1905; Angew. Chem. 2014, 126, 1932–1936;
- 11cQ. Hu, Z. Zhang, Y. Liu, T. Imamoto, W. Zhang, Angew. Chem. Int. Ed. 2015, 54, 2260–2264; Angew. Chem. 2015, 127, 2288–2292;
- 11dJ. Chen, Z. Zhang, B. Li, F. Li, Y. Wang, M. Zhao, I. D. Gridnev, T. Imamoto, W. Zhang, Nat. Commun. 2018, 9, 5000;
- 11eC. Liu, J. Yuan, J. Zhang, Z. Wang, Z. Zhang, W. Zhang, Org. Lett. 2018, 20, 108–111;
- 11fJ. Zhang, C. Liu, X. Wang, J. Chen, Z. Zhang, W. Zhang, Chem. Commun. 2018, 54, 6024–6027;
- 11gD. Fan, Y. Liu, J. Jia, Z. Zhang, Y. Liu, W. Zhang, Org. Lett. 2019, 21, 1042–1045;
- 11hJ. Zhang, J. Jia, X. Zeng, Y. Wang, Z. Zhang, I. D. Gridnev, W. Zhang, Angew. Chem. Int. Ed. 2019, 58, 11505–11512; Angew. Chem. 2019, 131, 11629–11636.
- 12
- 12aJ. Treutwein, G. Hilt, Angew. Chem. Int. Ed. 2008, 47, 6811–6813; Angew. Chem. 2008, 120, 6916–6919;
- 12bC.-H. Wei, S. Mannathan, C.-H. Cheng, J. Am. Chem. Soc. 2011, 133, 6942–6944;
- 12cQ.-A. Chen, D. K. Kim, V. M. Dong, J. Am. Chem. Soc. 2014, 136, 3772–3775;
- 12dJ. Yang, A. Rérat, Y. J. Lim, C. Gosmini, N. Yoshikai, Angew. Chem. Int. Ed. 2017, 56, 2449–2453; Angew. Chem. 2017, 129, 2489–2493;
- 12eS. Ghorai, S. S. Chirke, W.-B. Xu, J.-F. Chen, C. Li, J. Am. Chem. Soc. 2019, 141, 11430–11434.
- 13
- 13aM. J. Burk, J. E. Feaster, J. Am. Chem. Soc. 1992, 114, 6266–6267;
- 13bN. Yoshikawa, L. Tan, J. C. McWilliams, D. Ramasamy, R. Sheppard, Org. Lett. 2010, 12, 276–279;
- 13cM. Chang, S. Liu, K. Huang, X. Zhang, Org. Lett. 2013, 15, 4354–4357;
- 13dQ. Hu, Y. Hu, Y. Liu, Z. Zhang, Y. Liu, W. Zhang, Chem. Eur. J. 2017, 23, 1040–1043;
- 13eD. Fan, Y. Hu, F. Jiang, Z. Zhang, W. Zhang, Adv. Synth. Catal. 2018, 360, 2228–2232; Pd-catalyzed:
- 13fZ.-P. Chen, S.-B. Hu, J. Zhou, Y.-G. Zhou, ACS Catal. 2015, 5, 6086–6089;
- 13gZ.-P. Chen, S.-B. Hu, M.-W. Chen, Y.-G. Zhou, Org. Lett. 2016, 18, 2676–2679.
- 14C. J. Pilkington, A. Zanotti-Gerosa, Org. Lett. 2003, 5, 1273–1275.
- 15M. P. Magee, J. R. Norton, J. Am. Chem. Soc. 2001, 123, 1778–1779.
- 16A. K. Ghosh, J. Takayama, Y. Aubin, K. Ratia, R. Chaudhuri, Y. Baez, K. Sleeman, M. Coughlin, D. B. Nichols, D. C. Mulhearn, B. S. Prahakar, S. C. Baker, M. E. Johnson, A. D. Mesecar, J. Med. Chem. 2009, 52, 5228–5240.
- 17S. J. Stachel, R. Berger, A. B. Nomland, A. T. Ginnetti, D. V. Paone, D. Wang, V. Puri, H. Lange, J. Drott, J. Lu, J. Marcus, M. P. Dwyer, S. Suon, J. M. Uslaner, S. M. Smith, ACS Med. Chem. Lett. 2018, 9, 815–820.
- 18
- 18aD. K. Kim, J. Riedel, R. S. Kim, V. M. Dong, J. Am. Chem. Soc. 2017, 139, 10208–10211;
- 18bG. R. Morello, H. Zhong, P. J. Chirik, K. H. Hopmann, Chem. Sci. 2018, 9, 4977–4982.