A Genetically Encoded, Phage-Displayed Cyclic-Peptide Library
Xiaoshan Shayna Wang
Department of Chemistry, Texas A&M University, College Station, TX, 77843-3255 USA
These authors contributed equally to this work.
Search for more papers by this authorPeng-Hsun Chase Chen
Department of Chemistry, Texas A&M University, College Station, TX, 77843-3255 USA
These authors contributed equally to this work.
Search for more papers by this authorJ. Trae Hampton
Department of Chemistry, Texas A&M University, College Station, TX, 77843-3255 USA
Search for more papers by this authorJeffery M. Tharp
Department of Chemistry, Texas A&M University, College Station, TX, 77843-3255 USA
Search for more papers by this authorDr. Catrina A. Reed
Department of Chemistry, Texas A&M University, College Station, TX, 77843-3255 USA
Search for more papers by this authorDr. Sukant K. Das
Department of Chemistry, Texas A&M University, College Station, TX, 77843-3255 USA
Search for more papers by this authorDuen-Shian Wang
Department of Pharmaceutical Sciences, UNT Health Science Center, Fort Worth, TX, 76107 USA
Search for more papers by this authorHamed S. Hayatshahi
Department of Pharmaceutical Sciences, UNT Health Science Center, Fort Worth, TX, 76107 USA
Search for more papers by this authorProf. Dr. Yang Shen
Department of Electrical and Computer Engineering, Texas A&M University, College Station, TX, 77843-3218 USA
Search for more papers by this authorProf. Dr. Jin Liu
Department of Pharmaceutical Sciences, UNT Health Science Center, Fort Worth, TX, 76107 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Wenshe Ray Liu
Department of Chemistry, Texas A&M University, College Station, TX, 77843-3255 USA
Search for more papers by this authorXiaoshan Shayna Wang
Department of Chemistry, Texas A&M University, College Station, TX, 77843-3255 USA
These authors contributed equally to this work.
Search for more papers by this authorPeng-Hsun Chase Chen
Department of Chemistry, Texas A&M University, College Station, TX, 77843-3255 USA
These authors contributed equally to this work.
Search for more papers by this authorJ. Trae Hampton
Department of Chemistry, Texas A&M University, College Station, TX, 77843-3255 USA
Search for more papers by this authorJeffery M. Tharp
Department of Chemistry, Texas A&M University, College Station, TX, 77843-3255 USA
Search for more papers by this authorDr. Catrina A. Reed
Department of Chemistry, Texas A&M University, College Station, TX, 77843-3255 USA
Search for more papers by this authorDr. Sukant K. Das
Department of Chemistry, Texas A&M University, College Station, TX, 77843-3255 USA
Search for more papers by this authorDuen-Shian Wang
Department of Pharmaceutical Sciences, UNT Health Science Center, Fort Worth, TX, 76107 USA
Search for more papers by this authorHamed S. Hayatshahi
Department of Pharmaceutical Sciences, UNT Health Science Center, Fort Worth, TX, 76107 USA
Search for more papers by this authorProf. Dr. Yang Shen
Department of Electrical and Computer Engineering, Texas A&M University, College Station, TX, 77843-3218 USA
Search for more papers by this authorProf. Dr. Jin Liu
Department of Pharmaceutical Sciences, UNT Health Science Center, Fort Worth, TX, 76107 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Wenshe Ray Liu
Department of Chemistry, Texas A&M University, College Station, TX, 77843-3255 USA
Search for more papers by this authorGraphical Abstract
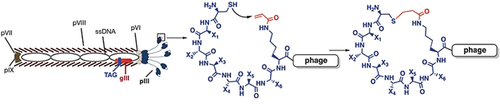
Using amber-codon suppression, Nϵ-acryloyl-lysine was genetically encoded in a phage-displayed peptide library for cyclization with a pre-installed cysteine. Selection from this phage-display library afforded cyclic peptides that bind TEV protease and histone deacetylase, HDAC8, much more strongly than their linear counterparts.
Abstract
Superior to linear peptides in biological activities, cyclic peptides are considered to have great potential as therapeutic agents. To identify cyclic-peptide ligands for therapeutic targets, phage-displayed peptide libraries in which cyclization is achieved by the covalent conjugation of cysteines have been widely used. To resolve drawbacks related to cysteine conjugation, we have invented a phage-display technique in which its displayed peptides are cyclized through a proximity-driven Michael addition reaction between a cysteine and an amber-codon-encoded Nϵ-acryloyl-lysine (AcrK). Using a randomized 6-mer library in which peptides were cyclized at two ends through a cysteine–AcrK linker, we demonstrated the successful selection of potent ligands for TEV protease and HDAC8. All selected cyclic peptide ligands showed 4- to 6-fold stronger affinity to their protein targets than their linear counterparts. We believe this approach will find broad applications in drug discovery.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201908713-sup-0001-misc_information.pdf2.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. K. Sato, M. Viswanathan, R. B. Kent, C. R. Wood, Curr. Opin. Biotechnol. 2006, 17, 638–642; G. L. Verdine, G. J. Hilinski, Methods Enzymol. 2012, 503, 3–33.
- 2N. Sternberg, R. H. Hoess, Proc. Natl. Acad. Sci. USA 1995, 92, 1609–1613; S. Li, S. Millward, R. Roberts, J. Am. Chem. Soc. 2002, 124, 9972–9973; N. K. Bashiruddin, H. Suga, Curr. Opin. Chem. Biol. 2015, 24, 131–138; E. T. Boder, K. D. Wittrup, Nat. Biotechnol. 1997, 15, 553–557; M. Yonezawa, N. Doi, Y. Kawahashi, T. Higashinakagawa, H. Yanagawa, Nucleic Acids Res. 2003, 31, e 118.
- 3T. Passioura, T. Katoh, Y. Goto, H. Suga, Annu. Rev. Biochem. 2014, 83, 727–753.
- 4T. Passioura, H. Suga, Chem. Commun. 2017, 53, 1931–1940.
- 5A. Kawamura, M. Munzel, T. Kojima, C. Yapp, B. Bhushan, Y. Goto, A. Tumber, T. Katoh, O. N. King, T. Passioura, L. J. Walport, S. B. Hatch, S. Madden, S. Muller, P. E. Brennan, R. Chowdhury, R. J. Hopkinson, H. Suga, C. J. Schofield, Nat. Commun. 2017, 8, 14773; J. Morimoto, Y. Hayashi, H. Suga, Angew. Chem. Int. Ed. 2012, 51, 3423–3427; Angew. Chem. 2012, 124, 3479–3483; A. Zorzi, K. Deyle, C. Heinis, Curr. Opin. Chem. Biol. 2017, 38, 24–29; N. Bionda, R. Fasan, Methods Mol. Biol. 2017, 1495, 57–76; A. E. Owens, I. de Paola, W. A. Hansen, Y. W. Liu, S. D. Khare, R. Fasan, J. Am. Chem. Soc. 2017, 139, 12559–12568.
- 6S. Chen, I. R. Rebollo, S. A. Buth, J. Morales-Sanfrutos, J. Touti, P. G. Leiman, C. Heinis, J. Am. Chem. Soc. 2013, 135, 6562–6569.
- 7D. J. Craik, Science 2006, 311, 1563–1564.
- 8D. J. Craik, D. P. Fairlie, S. Liras, D. Price, Chem. Biol. Drug Des. 2013, 81, 136–147.
- 9M. Katasara, T. Tselios, S. Deraos, G. Deraos, M. T. Matsoukas, E. Lazoura, J. Matsoukas, V. Apostolopoulos, Curr. Med. Chem. 2006, 13, 2221–2232.
- 10W. Xiao, Y. Wang, E. Y. Lau, J. Luo, N. Yao, C. Shi, L. Meza, H. Tseng, Y. Maeda, P. Kumaresan, R. Liu, F. C. Lightstone, Y. Takada, K. S. Lam, Mol. Cancer Ther. 2010, 9, 2714–2723; C. J. Hipolito, H. Suga, Curr. Opin. Chem. Biol. 2012, 16, 196–203; A. A. Vinogradov, Y. Yin, H. Suga, J. Am. Chem. Soc. 2019, 141, 4167–4181.
- 11K. Deyle, X. D. Kong, C. Heinis, Acc. Chem. Res. 2017, 50, 1866–1874.
- 12S. Palei, K. S. Becher, C. Nienberg, J. Jose, H. D. Mootz, ChemBioChem 2019, 20, 72–77; M. van Rosmalen, B. M. Janssen, N. M. Hendrikse, A. J. van der Linden, P. A. Pieters, D. Wanders, T. F. de Greef, M. Merkx, J. Biol. Chem. 2017, 292, 1477–1489; Y. Huang, M. M. Wiedmann, H. Suga, Chem. Rev. 2018 https://doi.org/10.1021/acs.chemrev.8b00430; A. Tavassoli, Curr. Opin. Chem. Biol. 2017, 38, 30–35.
- 13Z. Qian, P. Upadhyaya, D. Pei, Methods Mol. Biol. 2015, 1248, 39–53.
- 14R. Derda, M. R. Jafari, Protein Pept. Lett. 2018, 25, 1051–1075; S. Ng, R. Derda, Org. Biomol. Chem. 2016, 14, 5539–5545; M. R. Jafari, H. Yu, J. M. Wickware, Y. S. Lin, R. Derda, Org. Biomol. Chem. 2018, 16, 7588–7594.
- 15M. A. McLafferty, R. B. Kent, R. C. Ladner, W. Markland, Gene 1993, 128, 29–36; E. Koivunen, B. Wang, E. Ruoslahti, Bio-Technol. 1995, 13, 265–270; K. T. O'Neil, R. H. Hoess, S. A. Jackson, N. S. Ramachandran, S. A. Mousa, W. F. DeGrado, Proteins 1992, 14, 509–515.
- 16B. A. Desimmie, M. Humbert, E. Lescrinier, J. Hendrix, S. Vets, R. Gijsbers, R. M. Ruprecht, U. Dietrich, Z. Debyser, F. Christ, Mol. Ther. 2012, 20, 2064–2075; D. S. Choi, H. E. Jin, S. Y. Yoo, S. W. Lee, Bioconjugate Chem. 2014, 25, 216–223; S. C. Meyer, T. Gaj, I. Ghosh, Chem. Biol. Drug Des. 2006, 68, 3–10; B. A. Katz, Biochemistry 1995, 34, 15421–15429; J. M. Healy, O. Murayama, T. Maeda, K. Yoshino, K. Sekiguchi, M. Kikuchi, Biochemistry 1995, 34, 3948–3955.
- 17C. Heinis, T. Rutherford, S. Freund, G. Winter, Nat. Chem. Biol. 2009, 5, 502–507; S. S. Kale, C. Villequey, X. D. Kong, A. Zorzi, K. Deyle, C. Heinis, Nat. Chem. 2018, 10, 715–723; P. Diderich, D. Bertoldo, P. Dessen, M. M. Khan, I. Pizzitola, W. Held, J. Huelsken, C. Heinis, ACS Chem. Biol. 2016, 11, 1422–1427; V. Baeriswyl, H. Rapley, L. Pollaro, C. Stace, D. Teufel, E. Walker, S. Chen, G. Winter, J. Tite, C. Heinis, ChemMedChem 2012, 7, 1173–1176; A. Angelini, L. Cendron, S. Chen, J. Touati, G. Winter, G. Zanotti, C. Heinis, ACS Chem. Biol. 2012, 7, 817–821.
- 18C. Heinis, T. Rutherford, S. Freund, G. Winter, Nat. Chem. Biol. 2009, 5, 502–507; M. R. Jafari, L. Deng, P. I. Kitov, S. Ng, W. L. Matochko, K. F. Tjhung, A. Zeberoff, A. Elias, J. S. Klassen, R. Derda, ACS Chem. Biol. 2014, 9, 443–450; S. Bellotto, S. Chen, I. R. Rebollo, H. A. Wegner, C. Heinis, J. Am. Chem. Soc. 2014, 136, 5880–5883.
- 19I. Kather, C. A. Bippes, F. X. Schmid, J. Mol. Biol. 2005, 354, 666–678.
- 20F. Tian, M. L. Tsao, P. G. Schultz, J. Am. Chem. Soc. 2004, 126, 15962–15963; C. C. Liu, A. V. Mack, E. M. Brustad, J. H. Mills, D. Groff, V. V. Smider, P. G. Schultz, J. Am. Chem. Soc. 2009, 131, 9616–9617; C. C. Liu, H. Choe, M. Farzan, V. V. Smider, P. G. Schultz, Biochemistry 2009, 48, 8891–8898.
- 21J. M. Smith, J. R. Frost, R. Fasan, J. Org. Chem. 2013, 78, 3525–3531; T. Kawakami, T. Ishizawa, T. Fujino, P. C. Reid, H. Suga, H. Murakami, ACS Chem. Biol. 2013, 8, 1205–1214; Z. Xiang, H. Ren, Y. S. Hu, I. Coin, J. Wei, H. Cang, L. Wang, Nat. Methods 2013, 10, 885–888.
- 22Z. Xiang, V. K. Lacey, H. Ren, J. Xu, D. J. Burban, P. A. Jennings, L. Wang, Angew. Chem. Int. Ed. 2014, 53, 2190–2193; Angew. Chem. 2014, 126, 2222–2225.
- 23G. Srinivasan, C. M. James, J. A. Krzycki, Science 2002, 296, 1459–1462.
- 24Y. J. Lee, B. Wu, J. E. Raymond, Y. Zeng, X. Fang, K. L. Wooley, W. R. Liu, ACS Chem. Biol. 2013, 8, 1664–1670; Y. J. Lee, Y. Kurra, W. R. Liu, ChemBioChem 2016, 17, 456–461.
- 25X. S. Wang, Y. J. Lee, W. R. Liu, Chem. Commun. 2014, 50, 3176–3179.
- 26E. Kaya, M. Vrabel, C. Deiml, S. Prill, V. S. Fluxa, T. Carell, Angew. Chem. Int. Ed. 2012, 51, 4466–4469; Angew. Chem. 2012, 124, 4542–4545.
- 27A. Chakrabarti, I. Oehme, O. Witt, G. Oliveira, W. Sippl, C. Romier, R. J. Pierce, M. Jung, Trends Pharmacol. Sci. 2015, 36, 481–492; J. E. Lopez, S. E. Haynes, J. D. Majmudar, B. R. Martin, C. A. Fierke, J. Am. Chem. Soc. 2017, 139, 16222–16227; Y. Tian, V. W. Wong, G. L. Wong, W. Yang, H. Sun, J. Shen, J. H. Tong, M. Y. Go, Y. S. Cheung, P. B. Lai, M. Zhou, G. Xu, T. H. Huang, J. Yu, K. F. To, A. S. Cheng, H. L. Chan, Cancer Res. 2015, 75, 4803–4816.
- 28O. J. Ingham, R. M. Paranal, W. B. Smith, R. A. Escobar, H. Yueh, T. Snyder, J. A. Porco, Jr., J. E. Bradner, A. B. Beeler, ACS Med. Chem. Lett. 2016, 7, 929–932; I. Rettig, E. Koeneke, F. Trippel, W. C. Mueller, J. Burhenne, A. Kopp-Schneider, J. Fabian, A. Schober, U. Fernekorn, A. von Deimling, H. E. Deubzer, T. Milde, O. Witt, I. Oehme, Cell Death Dis. 2015, 6, e 1657; S. Balasubramanian, J. Ramos, W. Luo, M. Sirisawad, E. Verner, J. J. Buggy, Leukemia 2008, 22, 1026–1034; T. Heimburg, A. Chakrabarti, J. Lancelot, M. Marek, J. Melesina, A. T. Hauser, T. B. Shaik, S. Duclaud, D. Robaa, F. Erdmann, M. Schmidt, C. Romier, R. J. Pierce, M. Jung, W. Sippl, J. Med. Chem. 2016, 59, 2423–2435.
- 29D. Wegener, F. Wirsching, D. Riester, A. Schwienhorst, Chem. Biol. 2003, 10, 61–68.
- 30D. P. Dowling, S. G. Gattis, C. A. Fierke, D. W. Christianson, Biochemistry 2010, 49, 5048–5056; M. Marek, S. Kannan, A. T. Hauser, M. Moraes Mourao, S. Caby, V. Cura, D. A. Stolfa, K. Schmidtkunz, J. Lancelot, L. Andrade, J. P. Renaud, G. Oliveira, W. Sippl, M. Jung, J. Cavarelli, R. J. Pierce, C. Romier, PLoS Pathog. 2013, 9, e 1003645.
- 31A. Vannini, C. Volpari, P. Gallinari, P. Jones, M. Mattu, A. Carfi, R. De Francesco, C. Steinkuhler, S. Di Marco, EMBO Rep. 2007, 8, 879–884; A. A. Tabackman, R. Frankson, E. S. Marsan, K. Perry, K. E. Cole, J. Struct. Biol. 2016, 195, 373–378; J. R. Somoza, R. J. Skene, B. A. Katz, C. Mol, J. D. Ho, A. J. Jennings, C. Luong, A. Arvai, J. J. Buggy, E. Chi, J. Tang, B. C. Sang, E. Verner, R. Wynands, E. M. Leahy, D. R. Dougan, G. Snell, M. Navre, M. W. Knuth, R. V. Swanson, D. E. McRee, L. W. Tari, Structure 2004, 12, 1325–1334; N. J. Porter, D. W. Christianson, ACS Chem. Biol. 2017, 12, 2281–2286; C. Decroos, C. M. Bowman, J. A. Moser, K. E. Christianson, M. A. Deardorff, D. W. Christianson, ACS Chem. Biol. 2014, 9, 2157–2164; S. Banerjee, N. Adhikari, S. A. Amin, T. Jha, Eur. J. Med. Chem. 2019, 164, 214–240.
- 32W. Xuan, J. Li, X. Luo, P. G. Schultz, Angew. Chem. Int. Ed. 2016, 55, 10065–10068; Angew. Chem. 2016, 128, 10219–10222; W. Xuan, S. Shao, P. G. Schultz, Angew. Chem. Int. Ed. 2017, 56, 5096–5100; Angew. Chem. 2017, 129, 5178–5182; J. L. Furman, M. Kang, S. Choi, Y. Cao, E. D. Wold, S. B. Sun, V. V. Smider, P. G. Schultz, C. H. Kim, J. Am. Chem. Soc. 2014, 136, 8411–8417; N. Wang, B. Yang, C. Fu, H. Zhu, F. Zheng, T. Kobayashi, J. Liu, S. Li, C. Ma, P. G. Wang, Q. Wang, L. Wang, J. Am. Chem. Soc. 2018, 140, 4995–4999; X. H. Chen, Z. Xiang, Y. S. Hu, V. K. Lacey, H. Cang, L. Wang, ACS Chem. Biol. 2014, 9, 1956–1961.