The Three-Dimensional Dendrite-Free Zinc Anode on a Copper Mesh with a Zinc-Oriented Polyacrylamide Electrolyte Additive
Qi Zhang
Hunan Provincial Key Laboratory of Chemical Power Sources, College of Chemistry and Chemical Engineering, Central South University, Changsha, 410083 China
Search for more papers by this authorJingyi Luan
Hunan Provincial Key Laboratory of Chemical Power Sources, College of Chemistry and Chemical Engineering, Central South University, Changsha, 410083 China
Search for more papers by this authorLiang Fu
Collaborative Innovation Center for Green Development in Wuling Mountain Areas, Yangtze Normal University, Fuling, 408100 China
Search for more papers by this authorShengan Wu
Hunan Provincial Key Laboratory of Chemical Power Sources, College of Chemistry and Chemical Engineering, Central South University, Changsha, 410083 China
Search for more papers by this authorProf. Yougen Tang
Hunan Provincial Key Laboratory of Chemical Power Sources, College of Chemistry and Chemical Engineering, Central South University, Changsha, 410083 China
Search for more papers by this authorProf. Xiaobo Ji
Hunan Provincial Key Laboratory of Chemical Power Sources, College of Chemistry and Chemical Engineering, Central South University, Changsha, 410083 China
Search for more papers by this authorCorresponding Author
Prof. Haiyan Wang
Hunan Provincial Key Laboratory of Chemical Power Sources, College of Chemistry and Chemical Engineering, Central South University, Changsha, 410083 China
Search for more papers by this authorQi Zhang
Hunan Provincial Key Laboratory of Chemical Power Sources, College of Chemistry and Chemical Engineering, Central South University, Changsha, 410083 China
Search for more papers by this authorJingyi Luan
Hunan Provincial Key Laboratory of Chemical Power Sources, College of Chemistry and Chemical Engineering, Central South University, Changsha, 410083 China
Search for more papers by this authorLiang Fu
Collaborative Innovation Center for Green Development in Wuling Mountain Areas, Yangtze Normal University, Fuling, 408100 China
Search for more papers by this authorShengan Wu
Hunan Provincial Key Laboratory of Chemical Power Sources, College of Chemistry and Chemical Engineering, Central South University, Changsha, 410083 China
Search for more papers by this authorProf. Yougen Tang
Hunan Provincial Key Laboratory of Chemical Power Sources, College of Chemistry and Chemical Engineering, Central South University, Changsha, 410083 China
Search for more papers by this authorProf. Xiaobo Ji
Hunan Provincial Key Laboratory of Chemical Power Sources, College of Chemistry and Chemical Engineering, Central South University, Changsha, 410083 China
Search for more papers by this authorCorresponding Author
Prof. Haiyan Wang
Hunan Provincial Key Laboratory of Chemical Power Sources, College of Chemistry and Chemical Engineering, Central South University, Changsha, 410083 China
Search for more papers by this authorGraphical Abstract
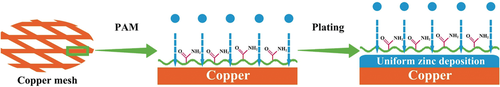
A dendrite-free zinc plating behavior was achieved by combining a Cu-Zn solid solution interface on a copper mesh skeleton and polyacrylamide electrolyte additive. The zinc ion has strong selective adsorption on the acyl group of polyacrylamide and can be transferred along the polymer chains, leading to the homogeneous zinc distribution.
Abstract
Rechargeable aqueous zinc-ion batteries have been considered as a promising candidate for next-generation batteries. However, the formation of zinc dendrites are the most severe problems limiting their practical applications. To develop stable zinc metal anodes, a synergistic method is presented that combines the Cu-Zn solid solution interface on a copper mesh skeleton with good zinc affinity and a polyacrylamide electrolyte additive to modify the zinc anode, which can greatly reduce the overpotential of the zinc nucleation and increase the stability of zinc deposition. The as-prepared zinc anodes show a dendrite-free plating/stripping behavior over a wide range of current densities. The symmetric cell using this dendrite-free anode can be cycled for more than 280 h with a very low voltage hysteresis (93.1 mV) at a discharge depth of 80 %. The high capacity retention and low polarization are also realized in Zn/MnO2 full cells.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201907830-sup-0001-misc_information.pdf2.1 MB | Supplementary |
| anie201907830-sup-0001-Movie_S1.mp41.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aY. Guo, H. Li, T. Zhai, Adv. Mater. 2017, 29, 1700007;
- 1bC. P. Grey, J. M. Tarascon, Nat. Mater. 2016, 16, 45–57.
- 2
- 2aL. Chen, J. L. Bao, X. Dong, D. G. Truhlar, Y. Wang, C. Wang, Y. Xia, ACS Energy Lett. 2017, 2, 1115–1121;
- 2bX. Wu, Y. Luo, M. Sun, J. Qian, Y. Cao, X. Ai, H. Yang, Nano Energy 2015, 13, 117–123;
- 2cP. Hu, T. Zhu, X. Wang, X. Wei, M. Yan, J. Li, W. Luo, W. Yang, W. Zhang, L. Zhou, Z. Zhou, L. Mai, Nano Lett. 2018, 18, 1758–1763;
- 2dD. Sun, G. Jin, H. Wang, P. Liu, Y. Ren, Y. Jiang, Y. Tang, X. Huang, J. Mater. Chem. A 2014, 2, 12999–13005.
- 3
- 3aJ. Huang, Z. Wang, M. Hou, X. Dong, Y. Liu, Y. Wang, Y. Xia, Nat. Commun. 2018, 9, 2906;
- 3bC. Zhu, G. Fang, J. Zhou, J. Guo, Z. Wang, C. Wang, J. Li, Y. Tang, S. Liang, J. Mater. Chem. A 2018, 6, 9677–9683;
- 3cB. S. Lee, S. Cui, X. Xing, H. Liu, X. Yue, V. Petrova, H. D. Lim, R. Chen, P. Liu, ACS Appl. Mater. Interfaces 2018, 10, 38928–38935.
- 4
- 4aL. L. Lu, Y. Zhang, Z. Pan, H. B. Yao, F. Zhou, S. H. Yu, Energy Storage Mater. 2017, 9, 31–38;
- 4bQ. Zhang, J. Luan, D. Sun, Y. Tang, H. Wang, Chem. Commun. 2019, 55, 6551–6554.
- 5Z. Kang, C. Wu, L. Dong, W. Liu, J. Mou, J. Zhang, Z. Chang, B. Jiang, G. Wang, F. Kang, C. Xu, ACS Sustainable Chem. Eng. 2019, 7, 3364–3371.
- 6G. Garcia, E. Ventosa, W. Schuhmann, ACS Appl. Mater. Interfaces 2017, 9, 18691–18698.
- 7
- 7aF. Wang, O. Borodin, T. Gao, X. Fan, W. Sun, F. Han, A. Faraone, J. A. Dura, K. Xu, C. Wang, Nat. Mater. 2018, 17, 543–549;
- 7bK. E. K. Sun, T. K. A. Hoang, T. N. L. Doan, Y. Yu, X. Zhu, Y. Tian, P. Chen, ACS Appl. Mater. Interfaces 2017, 9, 9681–9687;
- 7cL. P. Wang, N. W. Li, T. S. Wang, Y. X. Yin, Y. G. Guo, C. R. Wang, Electrochim. Acta 2017, 244, 172–177.
- 8
- 8aB. Tang, G. Fang, J. Zhou, L. Wang, Y. Lei, C. Wang, T. Lin, Y. Tang, S. Liang, Nano Energy 2018, 51, 579–587;
- 8bC. Xia, J. Guo, P. Li, X. Zhang, H. N. Alshareef, Angew. Chem. Int. Ed. 2018, 57, 3943–3948; Angew. Chem. 2018, 130, 4007–4012.
- 9A. Pei, G. Zheng, F. Shi, Y. Li, Y. Cui, Nano Lett. 2017, 17, 1132–1139.
- 10
- 10aG. D. Wilcox, P. J. Mitchell, J. Power Sources 1989, 28, 345–359;
- 10bB. G. Miller, T. W. Traut, R. Wolfenden, J. Am. Chem. Soc. 1998, 120, 2666–2667.
- 11K. M. Youssef, C. C. Koch, P. S. Fedkiw, Electrochim. Acta 2008, 54, 677–683.
- 12
- 12aQ. Li, S. Zhu, Y. Lu, Adv. Funct. Mater. 2017, 27, 1606422;
- 12bX. B. Cheng, T. Z. Hou, R. Zhang, H. J. Peng, C. Z. Zhao, J. Q. Huang, Q. Zhang, Adv. Mater. 2016, 28, 2888–2895;
- 12cC. P. Yang, Y. X. Yin, S. F. Zhang, N. W. Li, Y. G. Guo, Nat. Commun. 2015, 6, 8058.
- 13H. Li, Z. Liu, G. Liang, Y. Huang, Y. Huang, M. Zhu, Z. Pei, Q. Xue, Z. Tang, Y. Wang, B. Li, C. Zhi, ACS Nano 2018, 12, 3140–3148.
- 14
- 14aC. Martín, S. Merino, J. M. González-Domínguez, R. Rauti, L. Ballerini, M. Prato, E. Vázquez, Sci. Rep. 2017, 7, 10942;
- 14bL. Xie, X. Yang, Y. He, R. Yuan, Y. Chai, ACS Appl. Mater. Interfaces 2018, 10, 15200–15206.
- 15
- 15aB. Sharma, P. Bugga, L. R. Madison, A. I. Henry, M. G. Blaber, N. G. Greeneltch, N. Chiang, M. Mrksich, G. C. Schatz, R. P. Van Duyne, J. Am. Chem. Soc. 2016, 138, 13952–13959;
- 15bZ. Zhang, W. Yu, J. Wang, D. Luo, X. Qiao, X. Qin, T. Wang, Anal. Chem. 2017, 89, 1416–1420.
- 16W. Zeng, L. Wang, X. Peng, T. Liu, Y. Jiang, F. Qin, L. Hu, P. K. Chu, K. Huo, Y. Zhou, Adv. Energy Mater. 2018, 8, 1702314.
- 17Q. Zhang, J. Luan, Y. Tang, X. Ji, S. Wang, H. Wang, J. Mater. Chem. A 2018, 6, 18444–18448.
- 18S. F. Li, Z. W. Tang, Y. B. Tan, X. B. Yu, J. Phys. Chem. C 2012, 116, 1544–1549.
- 19
- 19aY. Liu, N. Zhang, L. Jiao, Z. Tao, J. Chen, Adv. Funct. Mater. 2015, 25, 214–220;
- 19bQ. Zhang, H. He, X. Huang, J. Yan, Y. Tang, H. Wang, Chem. Eng. J. 2018, 332, 57–65.
- 20P. S. da Silva, E. P. S. Schmitz, A. Spinelli, J. R. Garcia, J. Power Sources 2012, 210, 116–121.
- 21
- 21aK. Yan, Z. Lu, H. W. Lee, F. Xiong, P. C. Hsu, Y. Li, J. Zhao, S. Chu, Y. Cui, Nat. Energy 2016, 1, 16010;
- 21bM. Zhu, S. Li, B. Li, Y. Gong, Z. Du, S. Yang, Sci. Adv. 2019, 5, eaau 6264.
- 22A. A. Pankova, V. A. Blatov, G. D. Ilyushin, D. M. Proserpio, Inorg. Chem. 2013, 52, 13094–13107.
- 23R. F. Berger, P. L. Walters, S. Lee, R. Hoffmann, Chem. Rev. 2011, 111, 4522–4545.
- 24C. Liu, W. Zhou, J. Song, H. Liu, J. Qu, L. Guo, G. Song, C. P. Huang, J. Mater. Chem. A 2017, 5, 3145–3151.
- 25J. Zhao, J. Zhang, W. Yang, B. Chen, Z. Zhao, H. Qiu, S. Dong, X. Zhou, G. Cui, L. Chen, Nano Energy 2019, 57, 625–634.
- 26D. Zhang, J. Shi, Y. Qi, X. Wang, H. Wang, M. Li, S. Liu, C. Li, Adv. Sci. 2018, 5, 1801216.
- 27P. Zou, Y. Wang, S. W. Chiang, X. Wang, F. Kang, C. Yang, Nat. Commun. 2018, 9, 464.
- 28A. Koyama, K. Fukami, Y. Suzuki, A. Kitada, T. Sakka, T. Abe, K. Murase, M. Kinoshita, J. Phys. Chem. C 2016, 120, 24112–24120.
- 29S. S. Chi, X. G. Qi, Y. S. Hu, L. Z. Fan, Adv. Energy Mater. 2018, 8, 1702764.
- 30R. Zhang, X. R. Chen, X. Chen, X. B. Cheng, X. Q. Zhang, C. Yan, Q. Zhang, Angew. Chem. Int. Ed. 2017, 56, 7764–7768; Angew. Chem. 2017, 129, 7872–7876.
- 31Z. Zhao, J. Zhao, Z. Hu, J. Li, J. Li, Y. Zhang, C. Wang, G. Cui, Energy Environ. Sci. 2019, 12, 1938–1949.
- 32
- 32aH. He, Q. Gan, H. Wang, G. L. Xu, X. Zhang, D. Huang, F. Fu, Y. Tang, K. Amine, M. Shao, Nano Energy 2018, 44, 217–227;
- 32bD. Sun, D. Huang, H. Wang, G. L. Xu, X. Zhang, R. Zhang, Y. Tang, D. Abd Ei-Hady, W. Alshitari, A. Saad Al-Bogami, K. Amine, M. Shao, Nano Energy 2019, 61, 361–369.