Guest Binding via N−H⋅⋅⋅π Bonding and Kinetic Entrapment by an Inorganic Macrocycle
Alex J. Plajer
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW UK
Search for more papers by this authorDr. Felix J. Rizzuto
Department of Chemistry, McGill University, 801 Sherbrooke St. W, Montreal, Quebec, H3A 0B8 Canada
Search for more papers by this authorHao-Che Niu
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW UK
Search for more papers by this authorSanha Lee
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW UK
Search for more papers by this authorProf. Jonathan M. Goodman
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW UK
Search for more papers by this authorCorresponding Author
Prof. Dr. Dominic S. Wright
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW UK
Search for more papers by this authorAlex J. Plajer
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW UK
Search for more papers by this authorDr. Felix J. Rizzuto
Department of Chemistry, McGill University, 801 Sherbrooke St. W, Montreal, Quebec, H3A 0B8 Canada
Search for more papers by this authorHao-Che Niu
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW UK
Search for more papers by this authorSanha Lee
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW UK
Search for more papers by this authorProf. Jonathan M. Goodman
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW UK
Search for more papers by this authorCorresponding Author
Prof. Dr. Dominic S. Wright
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW UK
Search for more papers by this authorGraphical Abstract
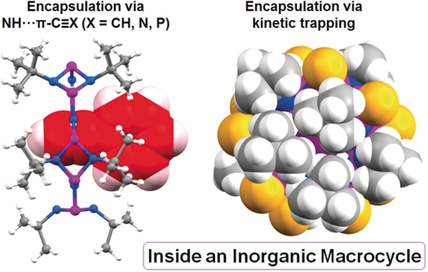
Ringing the Changes: The inorganic phosph(III)azane [{P(μ-NtBu)}2NH]5 macrocycle provides a unique H-bonding host environment for N−H⋅⋅⋅π bonding to alkynes, nitriles, and the PCO− anion. Oxidation of the PIII periphery with sulfur kinetically entraps halide ion guests, which cannot be abstracted even with powerful methylating agents.
Abstract
Modern supramolecular chemistry is overwhelmingly based on non-covalent interactions involving organic architectures. However, the question of what happens when you depart from this area to the supramolecular chemistry of structures based on non-carbon frameworks remains largely unanswered, and is an area that potentially provides new directions in molecular activation, host–guest chemistry, and biomimetic chemistry. In this work, we explore the unusual host–guest chemistry of the pentameric macrocycle [{P(μ-NtBu}2NH]5 with a range of anionic and neutral guests. The polar coordination site of this host promotes new modes of guest encapsulation via hydrogen bonding with the π systems of the unsaturated C≡C and C≡N bonds of acetylenes and nitriles as well as with the PCO− anion. Halide guests can be kinetically locked within the structure by oxidation of the phosphorus periphery by oxidation to PV. Our study underscores the future promise of p-block macrocyclic chemistry.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201905771-sup-0001-misc_information.pdf3.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J.-M. Lehn, Chem. Soc. Rev. 2007, 36, 151–160.
- 2J. W. Steed, J. L. Atwood, Supramolecular Chemistry, Wiley, Hoboken, 2013.
- 3H. J. Davis, R. J. Phipps, Chem. Sci. 2017, 8, 864–877.
- 4S. Russell Seidel, P. Stang, Acc. Chem. Res. 2002, 35, 972–983.
- 5S. Leininger, B. Olenyuk, P. J. Stang, Chem. Rev. 2000, 100, 853–908.
- 6J. R. Holst, A. Trewin, A. I. Cooper, Nat. Chem. 2010, 2, 915–920.
- 7C. J. Bruns, J. F. Stoddart, Acc. Chem. Res. 2014, 47, 2186–2199.
- 8R. S. Forgan, J.-P. Sauvage, J. F. Stoddart, Chem. Rev. 2011, 111, 5434–5464.
- 9T. K. Ronson, S. Zarra, S. P. Black, J. R. Nitschke, J. F. Stoddart, J. R. Nitschke, D. H. Simpson, P. Scott, M. Fujita, Chem. Commun. 2013, 49, 2476.
- 10Y.-R. Luo, Comprehensive Handbook of Chemical Bond Energies, CRC, Boca Raton, 2007.
10.1201/9781420007282 Google Scholar
- 11E. L. Doyle, L. Riera, D. S. Wright, Eur. J. Inorg. Chem. 2003, 3279–3289.
- 12P. A. Gale, N. Busschaert, C. J. E. Haynes, L. E. Karagiannidis, I. L. Kirby, Chem. Soc. Rev. 2014, 43, 205–241.
- 13M. Wenzel, J. R. Hiscock, P. A. Gale, Chem. Soc. Rev. 2012, 41, 480–520.
- 14C.-H. Chen, F. P. Gabbaï, Angew. Chem. Int. Ed. 2018, 57, 521–525; Angew. Chem. 2018, 130, 530–534.
- 15H. Klare, S. Hanft, J. M. Neudörfl, N. E. Schlörer, A. Griesbeck, B. Goldfuss, Chem. Eur. J. 2014, 20, 11847–11855.
- 16A. G. Schafer, J. M. Wieting, T. J. Fisher, A. E. Mattson, Angew. Chem. Int. Ed. 2013, 52, 11321–11324; Angew. Chem. 2013, 125, 11531–11534.
- 17C.-H. Chen, F. P. Gabbaï, Angew. Chem. Int. Ed. 2017, 56, 1799–1804; Angew. Chem. 2017, 129, 1825–1830.
- 18M. F. Hawthorne, Z. Zheng, Acc. Chem. Res. 1997, 30, 267–276.
- 19A. J. Martínez-Martínez, A. R. Kennedy, R. E. Mulvey, C. T. O'Hara, Science 2014, 346, 834–837.
- 20H.-C. Niu, A. J. Plajer, R. Garcia-Rodriguez, S. Singh, D. S. Wright, Chem. Eur. J. 2018, 24, 3073–3082.
- 21A. J. Plajer, R. García-Rodríguez, C. G. M. Benson, P. D. Matthews, A. D. Bond, S. Singh, L. H. Gade, D. S. Wright, Angew. Chem. Int. Ed. 2017, 56, 9087–9090; Angew. Chem. 2017, 129, 9215–9218.
- 22A. J. Plajer, H.-C. Niu, F. J. Rizzuto, D. S. Wright, Dalton Trans. 2018, 47, 6675–6678.
- 23A. Bashall, A. D. Bond, E. L. Doyle, F. García, S. Kidd, G. T. Lawson, M. C. Parry, M. McPartlin, A. D. Woods, D. S. Wright, Chem. Eur. J. 2002, 8, 3377.
10.1002/1521-3765(20020802)8:15<3377::AID-CHEM3377>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 24A. Nordheider, T. Chivers, R. Thirumoorthi, I. Vargas-Baca, J. D. Woollins, Chem. Commun. 2012, 48, 6346.
- 25A. Nordheider, T. Chivers, R. Thirumoorthi, K. S. Athukorala Arachchige, A. M. Z. Slawin, J. D. Woollins, I. Vargas-Baca, Dalton Trans. 2013, 42, 3291–3294.
- 26C. G. M. Benson, A. J. Plajer, R. García-Rodríguez, A. D. Bond, S. Singh, L. H. Gade, D. S. Wright, Chem. Commun. 2016, 52, 9683–9686.
- 27M. S. Balakrishna, D. J. Eisler, T. Chivers, Chem. Soc. Rev. 2007, 36, 650–664.
- 28M. S. Balakrishna, Dalton Trans. 2016, 45, 12252–12282.
- 29T. Chivers, R. S. Laitinen, Dalton Trans. 2017, 46, 1357–1367.
- 30S. Lee, C.-H. Chen, A. H. Flood, Nat. Chem. 2013, 5, 704–710.
- 31D. S. Kim, J. L. Sessler, Chem. Soc. Rev. 2015, 44, 532–546.
- 32J. L. Sessler, J. M. Davis, Acc. Chem. Res. 2001, 34, 989–997.
- 33K. Choi, A. D. Hamilton, Coord. Chem. Rev. 2003, 240, 101–110.
- 34J. M. Goicoechea, H. Grützmacher, Angew. Chem. Int. Ed. 2018, 57, 16968–16994; Angew. Chem. 2018, 130, 17214–17240.
- 35K. H. Møller, A. S. Hansen, H. G. Kjaergaard, J. Phys. Chem. A 2015, 119, 10988–10998.
- 36A. S. Hansen, L. Du, H. G. Kjaergaard, J. Phys. Chem. Lett. 2014, 5, 4225–4231.
- 37P. Thordarson, Chem. Soc. Rev. 2011, 40, 1305–1323.
- 38J.-Y. Le Questel, M. Berthelot, C. Laurence, J. Phys. Org. Chem. 2000, 13, 347-358.
- 39D. Mootz, A. Deeg, J. Am. Chem. Soc. 1992, 114, 5887–5888.
- 40T. E. Müller, D. M. P. Mingos, D. J. Williams, Chem. Commun. 1994, 1787–1788.
- 41D. A. Roberts, B. S. Pilgrim, J. R. Nitschke, Chem. Soc. Rev. 2018, 47, 626.
- 42B. Breiner, J. K. Clegg, J. R. Nitschke, Chem. Sci. 2011, 2, 51–56.
- 43S. J. Edwards, H. Valkenier, N. Busschaert, P. A. Gale, A. P. Davis, Angew. Chem. Int. Ed. 2015, 54, 4592–4596; Angew. Chem. 2015, 127, 4675–4679.
- 44I. A. Riddell, M. M. J. Smulders, J. K. Clegg, Y. R. Hristova, B. Breiner, J. D. Thoburn, J. R. Nitschke, Nat. Chem. 2012, 4, 751–756.
- 45V. Marcos, A. J. Stephens, J. Jaramillo-Garcia, A. L. Nussbaumer, S. L. Woltering, A. Valero, J.-F. Lemonnier, I. J. Vitorica-Yrezabal, D. A. Leigh, Science 2016, 352, 1555–1559.