The Miharamycins and Amipurimycin: their Structural Revision and the Total Synthesis of the Latter
Dr. Shengyang Wang
Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, 1200 Cai Lun Road, Shanghai, 201203 China
Search for more papers by this authorDr. Qingju Zhang
National Engineering Research Centre for Carbohydrate Synthesis, Jiangxi Normal University, Nanchang, 330022 China
Search for more papers by this authorYachen Zhao
State Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorProf. Jiansong Sun
National Engineering Research Centre for Carbohydrate Synthesis, Jiangxi Normal University, Nanchang, 330022 China
Search for more papers by this authorWenjia Kang
State Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorFei Wang
State Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorDr. Haixue Pan
State Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorProf. Gongli Tang
State Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Biao Yu
State Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorDr. Shengyang Wang
Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, 1200 Cai Lun Road, Shanghai, 201203 China
Search for more papers by this authorDr. Qingju Zhang
National Engineering Research Centre for Carbohydrate Synthesis, Jiangxi Normal University, Nanchang, 330022 China
Search for more papers by this authorYachen Zhao
State Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorProf. Jiansong Sun
National Engineering Research Centre for Carbohydrate Synthesis, Jiangxi Normal University, Nanchang, 330022 China
Search for more papers by this authorWenjia Kang
State Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorFei Wang
State Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorDr. Haixue Pan
State Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorProf. Gongli Tang
State Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Biao Yu
State Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorGraphical Abstract
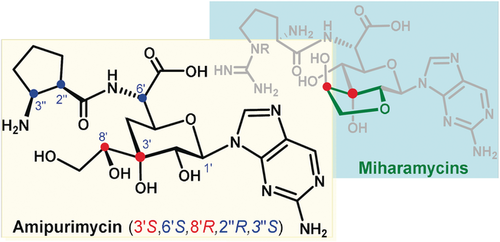
Final piece of the puzzle: The structures of amipurimycin and the miharamycins have been resolved using chemical synthesis and X-ray diffraction analysis. The stereochemistry of amipurimycin has been corrected. The miharamycins have a trans-fused dioxabicyclo[4.3.0]nonane sugar scaffold, which was previously assigned as being in the cis configuration.
Abstract
The structural puzzle of amipurimycin, a peptidyl nucleoside antibiotic, is solved by total synthesis and X-ray diffraction analysis, with the originally proposed configurations at C3′ and C8′ inverted and those at C6′, C2′′, and C3′′ corrected. A similar structural revision of the relevant miharamycins is proposed via chemical transformations and then validated by X-ray diffraction analysis. The miharamycins bear an unusual trans-fused dioxabicyclo[4.3.0]nonane sugar scaffold, which was previously assigned as being in the cis configuration.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201905723-sup-0001-misc_information.pdf3.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. Iwasa, T. Kishi, K. Matsuura, O. Wakae, J. Antibiot. 1977, 30, 1–10;
- 1bS. Harada, T. Kishi, J. Antibiot. 1977, 30, 11–16.
- 2T. Goto, Y. Toya, T. Ohgi, T. Kondo, Tetrahedron Lett. 1982, 23, 1271–1274.
- 3
- 3aT. Tsuruoka, H. Yumoto, N. Ezaki, T. Niida, Sci. Rep. Meiji Seika Kasha 1967, 9, 1–4;
- 3bT. Noguchi, Y. Yasuda, T. Niida, T. Shomura, Ann. Phytopath. Soc. Jpn. 1968, 34, 323–327.
- 4H. Seto, M. Koyama, H. Ogino, T. Tsuruoka, S. Inouye, N. Otake, Tetrahedron Lett. 1983, 24, 1805–1808.
- 5F. Marcelo, J. Jiménez-Barbero, J. Marrot, A. P. Rauter, P. Sinaÿ, Y. Blériot, Chem. Eur. J. 2008, 14, 10066–10073.
- 6
- 6aP. Garner, Tetrahedron Lett. 1984, 25, 5855–5858;
- 6bP. Garner, S. Ramakanth, J. Org. Chem. 1986, 51, 2609–2612;
- 6cP. Garner, J. U. Yoo, R. Sarabu, V. O. Kennedy, W. J. Youngs, Tetrahedron 1998, 54, 9303–9316;
- 6dK. Hara, H. Fujimoto, K. I. Sato, H. Hashimoto, J. Yoshimura, Carbohydr. Res. 1987, 159, 65–79;
- 6eA. P. Rauter, A. C. Fernandes, S. Czernecki, J. M. Valery, J. Org. Chem. 1996, 61, 3594–3598;
- 6fS. Czernecki, S. Franco, J. M. Valery, J. Org. Chem. 1997, 62, 4845–4847;
- 6gS. Czernecki, J. M. Valery, R. Wilkens, Bull. Chem. Soc. Jpn. 1996, 69, 1347–1351;
- 6hC. S. Stauffer, A. Datta, J. Org. Chem. 2008, 73, 4166–4174;
- 6iJ. Xue, Z. Guo, J. Carbohydr. Chem. 2008, 27, 51–69;
- 6jR. S. Mane, S. Ghosh, B. A. Chopade, O. Reiser, D. D. Dhavale, J. Org. Chem. 2011, 76, 2892–2895;
- 6kP. R. Markad, N. Kumbhar, D. D. Dhavale, Beilstein J. Org. Chem. 2016, 12, 1765–1771.
- 7
- 7aP. Garner, J. U. Yoo, R. Saraku, Tetrahedron 1992, 48, 4259–4270;
- 7bS. Czernecki, S. Franco, S. Horns, J.-M. Valery, Tetrahedron Lett. 1996, 37, 4003–4006;
- 7cG. Casiraghi, L. Colombo, G. Rassu, P. Spanu, J. Org. Chem. 1991, 56, 6523–6527.
- 8S. Wang, J. Sun, Q. Zhang, X. Cao, Y. Zhao, G. Tang, B. Yu, Angew. Chem. Int. Ed. 2018, 57, 2884–2888; Angew. Chem. 2018, 130, 2934–2938.
- 9Preliminary results on the biosynthesis of amipurimycin was recently reported, see: W.-J. Kang, H.-X. Pan, S. Wang, B. Yu, H. Hua, G.-L. Tang, Org. Lett. 2019, 21, 3148–3152.
- 10Graphic comparisons of the NMR spectroscopy data of the synthetic amipurimycin diastereoisomers with those reported for the natural product are provided in the Supporting Information.
- 11
- 11aM. Konishi, M. Nishio, K. Saitoh, T. Miyaki, T. Oki, H. Kawaguchi, J. Antibiot. 1989, 42, 1749–1755;
- 11bT. Oki, M. Hirano, K. Tomatsu, K.-I. Numata, H. Kamei, J. Antibiot. 1989, 42, 1756–1762;
- 11cT. Iwamoto, E. Tsujii, M. Ezaki, A. Fujie, S. Hashimoto, M. Okuhara, M. Kohsaka, H. Imanaka, K. Kawabata, Y. Inamoto, K. Sakane, J. Antibiot. 1990, 43, 1–7;
- 11dK. Kawabata, Y. Inamoto, K. Sakane, T. Iwamoto, S. Hashimoto, J. Antibiot. 1990, 43, 513–518.
- 12J. K. Khalaf, A. Datta, J. Org. Chem. 2004, 69, 387–390.
- 13
- 13aM. Sekiguchi, S. Obika, Y. Harada, T. Osaki, R. Somjing, Y. Mitsuoka, N. Shibata, M. Masaki, T. Imanishi, J. Org. Chem. 2006, 71, 1306–1316;
- 13bT. Osaki, S. Obika, Y. Harada, Y. Mitsuoka, K. Sugaya, M. Sekiguchi, S. Roongjang, T. Imanishi, Tetrahedron 2007, 63, 8977–8986.
- 14
- 14aY. C. Wu, J. Zhu, J. Org. Chem. 2008, 73, 9522–9524;
- 14bS. Manabe, Y. Ito, J. Org. Chem. 2013, 78, 4568–4572;
- 14cY. Liu, T. Song, W. Meng, Y. Xu, P. G. Wang, W. Zhao, Tetrahedron Lett. 2016, 57, 2758–2762.
- 15J. C. Lee, X. A. Lu, S. S. Kulkarni, Y. S. Wen, S. C. Hung, J. Am. Chem. Soc. 2004, 126, 476–477.
- 16W. Li, A. Silipo, A. Molinaro, B. Yu, Chem. Commun. 2015, 51, 6964–6967.
- 17
- 17aY. Li, Y. Yang, B. Yu, Tetrahedron Lett. 2008, 49, 3604–3608;
- 17bQ. Zhang, J. Sun, Y. Zhu, F. Zhang, B. Yu, Angew. Chem. Int. Ed. 2011, 50, 4933–4936; Angew. Chem. 2011, 123, 5035–5038;
- 17cF. Yang, G. Zhu, B. Yu, Chem. Commun. 2012, 48, 7097–7099;
- 17dS. Nie, W. Li, B. Yu, J. Am. Chem. Soc. 2014, 136, 4157–4160;
- 17eJ. Li, B. Yu, Angew. Chem. Int. Ed. 2015, 54, 6618–6621; Angew. Chem. 2015, 127, 6718–6721;
- 17fB. Yu, Acc. Chem. Res. 2018, 51, 507–516.
- 18
- 18aP. H. J. Carlsen, T. Katsuki, V. S. Martin, K. B. Sharpless, J. Org. Chem. 1981, 46, 3936–3938;
- 18bA. K. Singh, R. S. Varma, Tetrahedron Lett. 1992, 33, 2307–2310.
- 19J. R. Vaughan, Jr., J. Am. Chem. Soc. 1951, 73, 3547–3547.
- 20After submission of the present work, the biosynthetic gene clusters of amipurimycin and miharamycin were reported, see: A. J. Romo, T. Shiraishi, H. Ikeuchi, G.-M. Lin, Y. Geng, Y.-H. Lee, P. H. Liem, T. Ma, Y. Ogasawara, K. Shin-ya, M. Nishiyama, T. Kuzuyama, H.-w. Liu, J. Am. Chem. Soc. 2019, https://doi.org/10.1021/jacs.9b03021.