A Selection Rule for Hydrofluoroether Electrolyte Cosolvent: Establishing a Linear Free-Energy Relationship in Lithium–Sulfur Batteries
Graphical Abstract
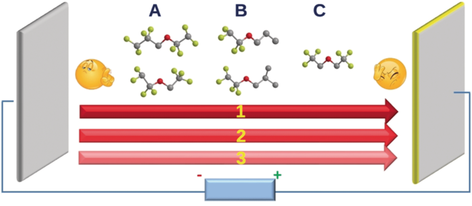
A–B–C easy as 1–2–3: High-performance batteries require tailored electrolyte cosolvents. The lithium-solvating ability of a series of hydrofluoroethers (HFEs), and the linear free-energy relationship between these solvents and lithium polysulfide (LiPS), are reported. HFE structures (A, B, and C) vary with respect to the location of fluoroalkyl groups and an ether oxygen atom. Key: lithium-solvating power (1), conductivity (2), LiPS dissolution (3).
Abstract
Hydrofluoroethers (HFEs) have been adopted widely as electrolyte cosolvents for battery systems because of their unique low solvating behavior. The electrolyte is currently utilized in lithium-ion, lithium–sulfur, lithium–air, and sodium-ion batteries. By evaluating the relative solvating power of different HFEs with distinct structural features, and considering the shuttle factor displayed by electrolytes that employ HFE cosolvents, we have established the quantitative structure–activity relationship between the organic structure and the electrochemical performance of the HFEs. Moreover, we have established the linear free-energy relationship between the structural properties of the electrolyte cosolvents and the polysulfide shuttle effect in lithium–sulfur batteries. These findings provide valuable mechanistic insight into the polysulfide shuttle effect in lithium–sulfur batteries, and are instructive when it comes to selecting the most suitable HFE electrolyte cosolvent for different battery systems.