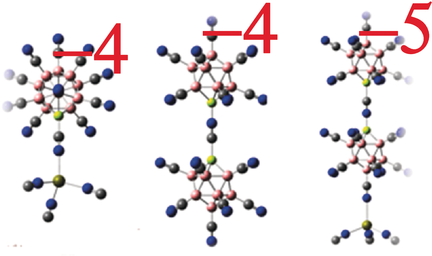
Stable Tetra- and Penta-Anions in the Gas Phase
Graphical Abstract
Abstract
The quest for stable gas-phase anions in highly negative charge states has been a great challenge. While multiply charged anions are stabilized in solids and liquids by compensating cations and solvation cells, respectively, stable anions containing less than a hundred atoms in the gas phase and capable of carrying charge beyond −3 is unknown. Here, we report the discovery of thermodynamically stable tetra- and penta-anions, containing less than 50 and 80 atoms, respectively, in the gas phase. A universal model is developed that explains their stability in terms of the synergy between closed shell, high electron affinity, and size and predicts new highly-charged anions by using the known charged clusters as building blocks. Synthesis of these species can open a new chapter in materials chemistry.