Acyl-Phosphide Anions via an Intermediate with Carbene Character: Reactions of K[PtBu2] and CO
Maotong Xu
Department of Chemistry, University of Toronto, 80 St. George St., Toronto, Ontario, M5S 3H6 Canada
Search for more papers by this authorDr. Andrew R. Jupp
Department of Chemistry, University of Toronto, 80 St. George St., Toronto, Ontario, M5S 3H6 Canada
Search for more papers by this authorCorresponding Author
Prof. Dr. Douglas W. Stephan
Department of Chemistry, University of Toronto, 80 St. George St., Toronto, Ontario, M5S 3H6 Canada
Search for more papers by this authorMaotong Xu
Department of Chemistry, University of Toronto, 80 St. George St., Toronto, Ontario, M5S 3H6 Canada
Search for more papers by this authorDr. Andrew R. Jupp
Department of Chemistry, University of Toronto, 80 St. George St., Toronto, Ontario, M5S 3H6 Canada
Search for more papers by this authorCorresponding Author
Prof. Dr. Douglas W. Stephan
Department of Chemistry, University of Toronto, 80 St. George St., Toronto, Ontario, M5S 3H6 Canada
Search for more papers by this authorGraphical Abstract
Abstract
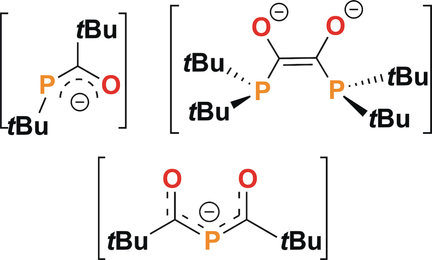
The analogy of the reactivity of group 1 phosphides to that of FLPs is further demonstrated by reactions with CO, affording a new synthetic route to acyl-phosphide anions. The reaction of [K(18-crown-6)][PtBu2] (1) with CO affords [(18-crown-6)K⋅THF2][Z-tBuP=C(tBu)O] (2⋅THF2) as the major product, and the minor product [K6(18-crown-6)][(tBu2PCO)2]3 (3). Species 2 reacts with either BPh3 or additional CO to give [K(18-crown-6)][(Ph3B)tBuPC(tBu)O] (4) and [K(18-crown-6)][(OCtBu)2P] (5), respectively. The acyl-phosphide anion 2 is thought to be formed by a photochemically induced radical process involving a transient species with triplet carbene character, prompting 1,2-tert-butyl group migration. A similar process is proposed for the subsequent reaction of 2 with CO to give 5.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201814562-sup-0001-misc_information.pdf1.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. W. Stephan, J. Am. Chem. Soc. 2015, 137, 10018–10032;
- 1bD. W. Stephan, G. Erker, Angew. Chem. Int. Ed. 2015, 54, 6400–6441; Angew. Chem. 2015, 127, 6498–6541;
- 1cD. W. Stephan, Science 2016, 354, aaf 7229;
- 1dJ. Lam, K. M. Szkop, E. Mosaferi, D. W. Stephan, Chem. Soc. Rev. 2019, https://doi.org/10.1039/C8CS00277K.
- 2
- 2aG. C. Welch, R. R. S. Juan, J. D. Masuda, D. W. Stephan, Science 2006, 314, 1124–1126;
- 2bJ. S. J. McCahill, G. C. Welch, D. W. Stephan, Angew. Chem. Int. Ed. 2007, 46, 4968–4971; Angew. Chem. 2007, 119, 5056–5059;
- 2cG. C. Welch, D. W. Stephan, J. Am. Chem. Soc. 2007, 129, 1880–1881;
- 2dP. Spies, G. Erker, G. Kehr, K. Bergander, R. Fröhlich, S. Grimme, D. W. Stephan, Chem. Commun. 2007, 5072–5074.
- 3
- 3aS. A. Weicker, D. W. Stephan, Bull. Chem. Soc. Jpn. 2015, 88, 1003–1016;
- 3b“Non-conventional Lewis Acids and Bases in Frustrated Lewis Pair Chemistry”: C. B. Caputo, D. W. Stephan in The Chemical Bond III: 100 years old and getting stronger, Vol. 171 (Ed.: ), Springer, 2017, pp. 1–29.
- 4
- 4aC. Walling, L. Bollyky, J. Am. Chem. Soc. 1964, 86, 3750–3752;
- 4bC. Walling, L. Bollyky, J. Am. Chem. Soc. 1961, 83, 2968–2969;
- 4cA. Berkessel, T. J. S. Schubert, T. N. Müller, J. Am. Chem. Soc. 2002, 124, 8693–8698.
- 5L. H. Slaugh, Tetrahedron 1966, 22, 1741–1746.
- 6L. H. Slaugh, J. Org. Chem. 1967, 32, 108–113.
- 7
- 7aJ. Spielmann, F. D. Buch, S. Harder, Angew. Chem. Int. Ed. 2008, 47, 9434–9438; Angew. Chem. 2008, 120, 9576–9580;
- 7bH. Bauer, M. Alonso, C. Färber, H. Elsen, J. Pahl, A. Causero, G. Ballmann, F. de Proft, S. Harder, Nat. Catal. 2018, 1, 40–47.
- 8
- 8aP. Jochmann, J. P. Davin, T. P. Spaniol, L. Maron, J. Okuda, Angew. Chem. Int. Ed. 2012, 51, 4452–4455; Angew. Chem. 2012, 124, 4528–4531;
- 8bD. Mukherjee, T. Hollerhage, V. Leich, T. P. Spaniol, U. Englert, L. Maron, J. Okuda, J. Am. Chem. Soc. 2018, 140, 3403–3411.
- 9M. T. Xu, A. R. Jupp, Z. W. Qu, D. W. Stephan, Angew. Chem. Int. Ed. 2018, 57, 11050–11054; Angew. Chem. 2018, 130, 11216–11220.
- 10S. Brand, H. Elsen, J. Langer, W. A. Donaubauer, F. Hampel, S. Harder, Angew. Chem. Int. Ed. 2018, 57, 14169–14173; Angew. Chem. 2018, 130, 14365–14369.
- 11
- 11aP. Jutzi, F.-W. Schröder, J. Organomet. Chem. 1970, 24, 1–5;
- 11bD. Seyferth, R. M. Weinstein, J. Am. Chem. Soc. 1982, 104, 5534–5535;
- 11cS. Murai, I. Ryu, J. Iriguchi, N. Sonoda, J. Am. Chem. Soc. 1984, 106, 2440–2442;
- 11dI. Ryu, Y. Hayama, A. Hirai, N. Sonoda, A. Orita, K. Ohe, S. Murai, J. Am. Chem. Soc. 1990, 112, 7061–7063;
- 11eH. Kai, K. Iwamoto, N. Chatani, S. Murai, J. Am. Chem. Soc. 1996, 118, 7634–7635;
- 11fI. Ryu, H. Yamamoto, N. Sonoda, S. Murai, Organometallics 1996, 15, 5459–5461;
- 11gK. Smith, G. A. El-Hiti, G. J. Pritchard, A. Hamilton, J. Chem. Soc. Perkin Trans. 1 1999, 2299–2303;
- 11hK. Iwamoto, N. Chatani, S. Murai, J. Org. Chem. 2000, 65, 7944–7948;
- 11iQ. Song, J. Chen, X. Jin, Z. Xi, J. Am. Chem. Soc. 2001, 123, 10419–10420;
- 11jQ. Song, Z. Li, J. Chen, C. Wang, Z. Xi, Org. Lett. 2002, 4, 4627–4629;
- 11kT. Fukuyama, T. Totoki, I. Ryu, Org. Lett. 2014, 16, 5632–5635.
- 12
- 12aM. S. Kharasch, O. Reinmuth, Grignard reactions of nonmetallic substances, Prentice-Hall, New York, 1954;
- 12bW. J. J. M. Sprangers, A. P. van Swieten, R. Louw, Tetrahedron Lett. 1974, 15, 3377–3378.
10.1016/S0040-4039(01)91911-2 Google Scholar
- 13
- 13aP. Jutzi, F. W. Schröder, Angew. Chem. Int. Ed. Engl. 1971, 10, 339; Angew. Chem. 1971, 83, 334;
- 13bA. Orita, M. Fukudome, K. Ohe, S. Murai, J. Org. Chem. 1994, 59, 477–481;
- 13cH. Kai, M. Yamauchi, S. Murai, Tetrahedron Lett. 1997, 38, 9027–9030;
- 13dH. Kai, A. Orita, S. Murai, Synth. Commun. 1998, 28, 1989–2000.
- 14F. F. Puschmann, D. Stein, D. Heift, C. Hendriksen, Z. A. Gal, H. F. Grützmacher, H. Grützmacher, Angew. Chem. Int. Ed. 2011, 50, 8420–8423; Angew. Chem. 2011, 123, 8570–8574.
- 15G. Becker, W. Becker, M. Schmidt, W. Schwarz, M. Westerhausen, Z. Anorg. Allg. Chem. 1991, 605, 7–23.
- 16
- 16aG. Müller, M. Zalibera, G. Gescheidt, A. Rosenthal, G. Santiso-Quinones, K. Dietliker, H. Grützmacher, Macromol. Rapid Commun. 2015, 36, 553–557;
- 16bD. A. Huber, A. Kuschel, T. Ott, G. Santiso-Quinones, D. Stein, J. Brauer, R. Kissner, F. Krumeich, H. Schonberg, J. Levalois-Grützmacher, H. Grützmacher, Angew. Chem. Int. Ed. 2012, 51, 4648–4652; Angew. Chem. 2012, 124, 4726–4730.
- 17
- 17aG. Becker, M. Niemeyer, O. Mundt, W. Schwarz, M. Westerhausen, M. W. Ossberger, P. Mayer, H. Nöth, Z. Zhong, P. J. Dijkstra, J. Feijen, Z. Anorg. Allg. Chem. 2004, 630, 2605–2621;
- 17bG. Becker, M. Rössler, G. Uhl, Z. Anorg. Allg. Chem. 1982, 495, 73–88.
- 18A. R. Jupp, G. Trott, E. Payen de la Garanderie, J. D. G. Holl, D. Carmichael, J. M. Goicoechea, Chem. Eur. J. 2015, 21, 8015–8018.
- 19E. Despagnet, H. Gornitzka, A. B. Rozhenko, W. W. Schoeller, D. Bourissou, G. Bertrand, Angew. Chem. Int. Ed. 2002, 41, 2835–2837;
10.1002/1521-3773(20020802)41:15<2835::AID-ANIE2835>3.0.CO;2-8 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 2959–2961.
- 20
- 20aA. E. Keating, M. A. Garcia-Garibay, K. N. Houk, J. Phys. Chem. A 1998, 102, 8467–8476;
- 20bX. Cattoën, H. Gornitzka, F. S. Tham, K. Miqueu, D. Bourissou, G. Bertrand, Eur. J. Org. Chem. 2007, 912–917;
- 20cD. L. Reid, J. Warkentin, J. Chem. Soc. Perkin Trans. 2 2000, 1980–1983.