Enantioconvergent Cross-Couplings of Alkyl Electrophiles: The Catalytic Asymmetric Synthesis of Organosilanes
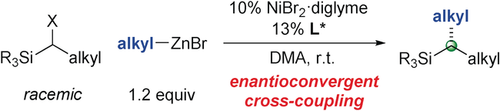
Graphical Abstract
Enantioconvergent cross-couplings with electrophiles that lack a directing group or a proximal p/π orbital were achieved for the first time. Specifically, a chiral nickel catalyst can accomplish Negishi reactions of racemic α-halosilanes with alkylzinc reagents with good enantioselectivity under simple and mild conditions, thereby providing access to enantioenriched organosilanes, an important class of target molecules.
Abstract
Metal-catalyzed enantioconvergent cross-coupling reactions of alkyl electrophiles are emerging as a powerful tool in asymmetric synthesis. To date, high enantioselectivity has been limited to couplings of electrophiles that bear a directing group or a proximal p/π orbital. Herein, we demonstrate for the first time that enantioconvergent cross-couplings can be achieved with electrophiles that lack such features; specifically, we establish that a chiral nickel catalyst can accomplish Negishi reactions of racemic α-halosilanes with alkylzinc reagents with good enantioselectivity under simple and mild conditions, thereby providing access to enantioenriched organosilanes, an important class of target molecules.