Catalytic Enantioselective Ynamide Additions to Isatins: Concise Access to Oxindole Alkaloids
Max Moskowitz
Department of Chemistry, Georgetown University, 37th and O Streets, Washington, DC, 20057 USA
Search for more papers by this authorCorresponding Author
Prof. Christian Wolf
Department of Chemistry, Georgetown University, 37th and O Streets, Washington, DC, 20057 USA
Search for more papers by this authorMax Moskowitz
Department of Chemistry, Georgetown University, 37th and O Streets, Washington, DC, 20057 USA
Search for more papers by this authorCorresponding Author
Prof. Christian Wolf
Department of Chemistry, Georgetown University, 37th and O Streets, Washington, DC, 20057 USA
Search for more papers by this authorGraphical Abstract
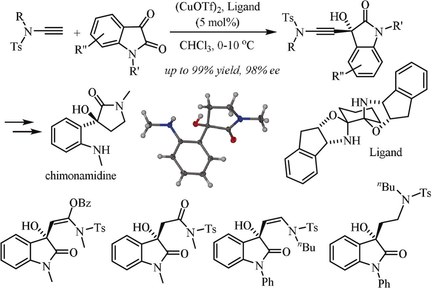
Terminal ynamide additions to isatins are accomplished with high yields and enantioselectivities by base-free CuI-bisoxazolidine catalysis. This approach provides efficient access to chimonamidines and 3-hydroxyindolinones carrying a tetrasubstituted chiral center with a synthetically versatile ynamide moiety.
Abstract
The highly enantioselective addition of terminal ynamides to a variety of isatins, catalyzed by a bisoxazolidine copper complex under mild, base-free reaction conditions, is described. The reaction is broad in scope, scalable, applicable to unprotected isatins, and provides efficient access to 3-hydroxyoxindoles carrying a tetrasubstituted chiral center with excellent yields and enantioselectivities. Synthetically versatile, multifunctional 3-hydroxyindolinones are obtained by hydration, partial hydrogenation, or hydroxyacyloxylation of the ynamide moiety at room temperature and exhaustive hydrogenation followed by reductive detosylation and spontaneous cyclization affords cinchonamidine alkaloids.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201814074-sup-0001-misc_information.pdf2.8 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. Ishimaru, N. Shibata, T. Horikawa, N. Yasuda, S. Nakamura, T. Toru, M. Shiro, Angew. Chem. Int. Ed. 2008, 47, 4157; Angew. Chem. 2008, 120, 4225;
- 1bS. Ma, X. Han, S. Krishnan, S. C. Virgil, B. M. Stoltz, Angew. Chem. Int. Ed. 2009, 48, 8037; Angew. Chem. 2009, 121, 8181;
- 1cT. Bui, S. Syed, C. F. Barbas III, J. Am. Chem. Soc. 2009, 131, 8758;
- 1dA. P. Antonchick, C. Gerding-Reimers, M. Catarinella, M. Schürmann, H. Preut, S. Ziegler, D. Rauh, H. Waldmann, Nat. Chem. 2010, 2, 735;
- 1eB. Tan, N. R. Candeias, C. F. Barbas, Nat. Chem. 2011, 3, 473;
- 1fC. Guo, J. Song, J.-Z. Huang, P.-H. Chen, S.-W. Luo, L.-Z. Gong, Angew. Chem. Int. Ed. 2012, 51, 1046; Angew. Chem. 2012, 124, 1070;
- 1gL. Wu, L. Falivene, E. Drinkel, S. Grant, A. Linden, L. Cavallo, R. Dorta, Angew. Chem. Int. Ed. 2012, 51, 2870; Angew. Chem. 2012, 124, 2924;
- 1hW. Xie, G. Jiang, H. Liu, J. Hu, X. Pan, H. Zhang, X. Wan, Y. Lai, D. Mae, Angew. Chem. Int. Ed. 2013, 52, 12924; Angew. Chem. 2013, 125, 13162;
- 1iH. Mitsunuma, M. Shibasaki, M. Kanai, S. Matsunaga, Angew. Chem. Int. Ed. 2012, 51, 5217; Angew. Chem. 2012, 124, 5307;
- 1jL. Zong, S. Du, K. F. Chin, C. Wang, C.-H. Tan, Angew. Chem. Int. Ed. 2015, 54, 9390; Angew. Chem. 2015, 127, 9522;
- 1kJ. Zhao, B. Fang, W. Luo, X. Hao, X. Liu, L. Lin, X. Feng, Angew. Chem. Int. Ed. 2015, 54, 241; Angew. Chem. 2015, 127, 243;
- 1lJ.-S. Yu, F.-M. Liao, W.-M. Gao, K. Liao, R.-L. Zuo, J. Zhou, Angew. Chem. Int. Ed. 2015, 54, 7381; Angew. Chem. 2015, 127, 7489;
- 1mO. D. Engl, S. P. Fritz, H. Wennemers, Angew. Chem. Int. Ed. 2015, 54, 8193; Angew. Chem. 2015, 127, 8311;
- 1nM.-Y. Wu, W.-W. He, X.-Y. Liu, B. Tan, Angew. Chem. Int. Ed. 2015, 54, 9409; Angew. Chem. 2015, 127, 9541;
- 1oP. Biswas, S. Paul, J. Guin, Angew. Chem. Int. Ed. 2016, 55, 7756; Angew. Chem. 2016, 128, 7887;
- 1pM. G. Sankar, M. Garcia-Castro, C. Golz, C. Strohmann, K. Kumar, Angew. Chem. Int. Ed. 2016, 55, 9709; Angew. Chem. 2016, 128, 9861;
- 1qW. Kong, Q. Wang, J. Zhu, Angew. Chem. Int. Ed. 2016, 55, 9714; Angew. Chem. 2016, 128, 9866;
- 1rK. Balaraman, C. Wolf, Angew. Chem. Int. Ed. 2017, 56, 1390; Angew. Chem. 2017, 129, 1411;
- 1sK. Balaraman, R. Ding, C. Wolf, Adv. Synth. Catal. 2017, 359, 4165;
- 1tR. Ding, C. Wolf, Org. Lett. 2018, 20, 892.
- 2For selected examples, see:
- 2aD. Sano, K. Nagata, T. Itoh, Org. Lett. 2008, 10, 1593;
- 2bT. Itoh, H. Ishikawa, Y. Hayashi, Org. Lett. 2009, 11, 3854;
- 2cW.-B. Chen, X.-L. Du, L.-F. Cun, X.-M. Zhang, W.-C. Yuan, Tetrahedron 2010, 66, 1441;
- 2dQ. Guo, M. Bhanushali, C.-G. Zhao, Angew. Chem. Int. Ed. 2010, 49, 9460; Angew. Chem. 2010, 122, 9650;
- 2eN. Hara, S. Nakamura, Y. Funahashi, N. Shibata, Adv. Synth. Catal. 2011, 353, 2976;
- 2fY. Yang, F. Moinodeen, W. Chin, T. Ma, Z. Jiang, C.-H. Tan, Org. Lett. 2012, 14, 4762;
- 2gB. Zhu, W. Zhang, R. Lee, Z. Han, W. Yang, D. Tan, K.-W. Huang, Z. Jiang, Angew. Chem. Int. Ed. 2013, 52, 6666; Angew. Chem. 2013, 125, 6798;
- 2hR. Ding, C. Wolf, J. Org. Chem. 2017, 82, 1273;
- 2iU. V. Subba Reddy, M. Chennapuram, K. Seki, C. Ski, B. Anusha, E. Kwon, Y. Okuyama, K. Uwai, M. Tokiwa, M. Takeshita, H. Nakano, Eur. J. Org. Chem. 2017, 3874;
- 2jM. Moskowitz, K. Balaraman, C. Wolf, J. Org. Chem. 2018, 83, 1661; For asymmetric synthesis of isoindolinones, see
- 2kT. Li, C. Zhou, X. Yan, J. Wang, Angew. Chem. Int. Ed. 2018, 57, 4048; Angew. Chem. 2018, 130, 4112.
- 3
- 3aN. Xu, D.-W. Gu, J. Zi, X.-Y. Wu, X.-X. Guo, Org. Lett. 2016, 18, 2439;
- 3bQ. Chen, Y. Tang, T. Huang, X. Liu, L. Lin, X. Feng, Angew. Chem. Int. Ed. 2016, 55, 5286; Angew. Chem. 2016, 128, 5372;
- 3cL. Chen, G. Huang, M. Liu, Z. Huang, F.-R. Chen, Adv. Synth. Catal. 2018, 360, 3497.
- 4
- 4aC. A. Zificsak, J. A. Mulder, R. P. Hsung, C. Rameshkumar, L.-L. Wei, Tetrahedron 2001, 57, 7575;
- 4bG. Evano, A. Coste, K. Jouvin, Angew. Chem. Int. Ed. 2010, 49, 2840; Angew. Chem. 2010, 122, 2902;
- 4cK. A. DeKorver, H. Li, A. G. Lohse, R. Hayashi, Z. Lu, Y. Zhang, R. P. Hsung, Chem. Rev. 2010, 110, 5064;
- 4dX.-N. Wang, H.-S. Yeom, L.-C. Fang, S. He, Z.-X. Ma, B. L. Kedrowski, R. P. Hsung, Acc. Chem. Res. 2014, 47, 560;
- 4eH. V. Adcock, E. Chatzopoulou, P. W. Davies, Angew. Chem. Int. Ed. 2015, 54, 15525; Angew. Chem. 2015, 127, 15745;
- 4fR. N. Straker, M. K. Majhail, M. C. Willis, Chem. Sci. 2017, 8, 7963;
- 4gX. Zeng, J. Li, C. K. Ng, G. B. Hammond, B. Xu, Angew. Chem. Int. Ed. 2018, 57, 2924; Angew. Chem. 2018, 130, 2974;
- 4hY. Gao, G. Wu, Q. Zhou, J. Wang, Angew. Chem. Int. Ed. 2018, 57, 2716; Angew. Chem. 2018, 130, 2746;
- 4iS. J. Mansfield, K. E. Christensen, A. L. Thompson, K. Ma, M. W. Jones, A. Mekareeya, E. A. Anderson, Angew. Chem. Int. Ed. 2017, 56, 14428; Angew. Chem. 2017, 129, 14620;
- 4jN. Marien, B. N. Reddy, F. De Vleeschouwer, S. Goderis, K. Van Hecke, G. Verniest, Angew. Chem. Int. Ed. 2018, 57, 5660; Angew. Chem. 2018, 130, 5762.
- 5
- 5aT. Hamada, X. Ye, S. S. Stahl, J. Am. Chem. Soc. 2008, 130, 833;
- 5bS. J. Mansfield, C. D. Campbell, M. W. Jones, E. A. Anderson, Chem. Commun. 2015, 51, 3316;
- 5cY. Tu, X. Zeng, H. Wang, J. Zhao, Org. Lett. 2018, 20, 280.
- 6
- 6aX.-N. Wang, R. P. Hsung, R. Qi, S. K. Fox, M.-C. Lv, Org. Lett. 2013, 15, 2514;
- 6bX.-N. Wang, R. P. Hsung, S. K. Fox, M.-C. Lv, R. Qi, Heterocycles 2014, 88, 1233;
- 6cA. M. Cook, C. Wolf, Chem. Commun. 2014, 50, 3151;
- 6dA. M. Cook, C. Wolf, Tetrahedron Lett. 2015, 56, 2377;
- 6eA. M. Cook, C. Wolf, Angew. Chem. Int. Ed. 2016, 55, 2929; Angew. Chem. 2016, 128, 2982;
- 6fJ. Zhao, J. Yang, C. Wang, S. Xu, Angew. Chem. Int. Ed. 2019, 58, 1382; Angew. Chem. 2019, 131, 1396.
- 7H. A. Laub, G. Evano, H. Mayr, Angew. Chem. Int. Ed. 2014, 53, 4968; Angew. Chem. 2014, 126, 5068.
- 8
- 8aC. Wolf, S. Liu, J. Am. Chem. Soc. 2006, 128, 10996;
- 8bS. Liu, C. Wolf, Org. Lett. 2007, 9, 2965;
- 8cS. Liu, C. Wolf, Org. Lett. 2008, 10, 1831;
- 8dK. Yearick Spangler, C. Wolf, Org. Lett. 2009, 11, 4724;
- 8eH. Xu, C. Wolf, Synlett 2010, 2765;
- 8fC. Wolf, M. Moskowitz, J. Org. Chem. 2011, 76, 6372;
- 8gH. Xu, C. Wolf, Angew. Chem. Int. Ed. 2011, 50, 12249; Angew. Chem. 2011, 123, 12457;
- 8hH. Xu, C. Wolf, Chem. Commun. 2010, 46, 8026;
- 8iC. Wolf, P. Zhang, Adv. Synth. Catal. 2011, 353, 760.
- 9C. Wolf, H. Xu, Chem. Commun. 2011, 47, 3339.
- 10Under these conditions, phenylacetylene addition to isatin 3 occurs smoothly to give 99 % yield of racemic product.
- 11For examples of ynamide transformations via keteniminium ion intermediates, see
- 11aM. Lecomte, G. Evano, Angew. Chem. Int. Ed. 2016, 55, 4547; Angew. Chem. 2016, 128, 4623;
- 11bL. L. Baldassari, A. de la Torre, J. Li, D. S. Lüdtke, N. Maulide, Angew. Chem. Int. Ed. 2017, 56, 15723; Angew. Chem. 2017, 129, 15929;
- 11cD. V. Patil, S. W. Kim, Q. H. Nguyen, H. Kim, S. Wang, T. Hoang, S. Shin, Angew. Chem. Int. Ed. 2017, 56, 3670; Angew. Chem. 2017, 129, 3724;
- 11dA. Pinto, D. Kaiser, B. Maryasin, G. Di Mauro, L. Gonzalez, N. Maulide, Chem. Eur. J. 2018, 24, 2515.
- 12
- 12aK. C. M. Kurtz, R. P. Hsung, Y. Zhang, Org. Lett. 2006, 8, 231;
- 12bL. You, Z. F. Al-Rashid, S. K. Ghosh, G. Li, R. P. Hsung, Synlett 2007, 1656;
- 12cX.-N. Wang, Z.-X. Ma, J. Deng, R. P. Hsung, Tetrahedron Lett. 2015, 56, 3463;
- 12dY. Yabuuchi, T. Kuzuguchi, T. Yoshimura, J.-i. Matsuo, Org. Lett. 2016, 18, 4951; For asymmetric catalysis with enamides:
- 12eR. Matsubara, S. Kobayashi, Acc. Chem. Res. 2008, 41, 292.
- 13The bisoxazolidine ligand undergoes N,N′-coordination to CuI.
- 14
- 14aP. Valenta, P. J. Carroll, P. J. Walsh, J. Am. Chem. Soc. 2010, 132, 14179;
- 14bK. Gopalaiah, H. B. Kagan, Chem. Rev. 2011, 111, 4599.
- 15A. Siva Reddy, K. C. K. Swamy, Angew. Chem. Int. Ed. 2017, 56, 6984; Angew. Chem. 2017, 129, 7088.
- 16
- 16aD. L. Smith, W. R. F. Goundry, H. W. Lam, Chem. Commun. 2012, 48, 1505;
- 16bS. Xu, J. Liu, D. Hu, X. Bi, Green Chem. 2015, 17, 184.
- 17CCDC 1882888 and 1882889 (16, 23) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre