Transfer Hydrocyanation of α- and α,β-Substituted Styrenes Catalyzed by Boron Lewis Acids
Patrizio Orecchia
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
These authors contributed equally to this work.
Search for more papers by this authorDr. Weiming Yuan
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. Martin Oestreich
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Search for more papers by this authorPatrizio Orecchia
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
These authors contributed equally to this work.
Search for more papers by this authorDr. Weiming Yuan
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. Martin Oestreich
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Search for more papers by this authorGraphical Abstract
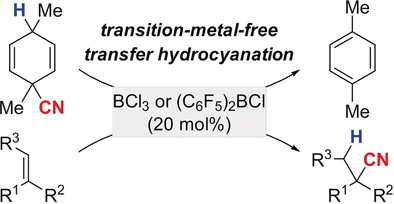
Acid-free! Bench-stable cyclohexa-1,4-diene-based hydrogen cyanide (HCN) surrogates are reported to engage in transfer hydrocyanation of alkenes upon treatment with certain boron Lewis acids. The success also depends on the equilibrium position between the intermediate isocyano- and cyanoborate isomers. Tertiary nitriles are obtained with exclusive Markovnikov selectivity.
Abstract
A straightforward gram-scale preparation of cyclohexa-1,4-diene-based hydrogen cyanide (HCN) surrogates is reported. These are bench-stable but formally release HCN and rearomatize when treated with Lewis acids. For BCl3, the formation of the isocyanide adduct [(CN)BCl3]− and the corresponding Wheland complex was verified by mass spectrometry. In the presence of 1,1-di- and trisubstituted alkenes, transfer of HCN from the surrogate to the C−C double bond occurs, affording highly substituted nitriles with Markovnikov selectivity. The success of this transfer hydrocyanation depends on the Lewis acid employed; catalytic amounts of BCl3 and (C6F5)2BCl are shown to be effective while B(C6F5)3 and BF3⋅OEt2 are not.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201813853-sup-0001-misc_information.pdf2.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. V. Rajanbabu, Org. React. 2011, 75, 1–73;
- 1bL. Bini, C. Müller, D. Vogt, Chem. Commun. 2010, 46, 8325–8334;
- 1cL. Bini, C. Müller, D. Vogt, ChemCatChem 2010, 2, 590–608;
- 1dC. A. Tolman, J. Chem. Educ. 1986, 63, 199–201;
- 1eC. A. Tolman, R. J. McKinney, W. C. Seidel, J. D. Druliner, W. R. Stevens, Adv. Catal. 1985, 33, 1–46.
- 2Exposure to 25–75 ppm for 1 h already produces serious symptoms: Hydrogen Cyanide in National Institute for Occupational Safety and Health (NIOSH).
- 3A. M. Nauth, T. Opatz, Org. Biomol. Chem. 2019, 17, 11–23.
- 4
- 4aM. J. Baker, P. G. Pringle, J. Chem. Soc. Chem. Commun. 1991, 1292–1293;
- 4bM. Yan, Q.-Y. Xu, A. S. C. Chan, Tetrahedron: Asymmetry 2000, 11, 845–849;
- 4cM. de Greef, B. Breit, Angew. Chem. Int. Ed. 2009, 48, 551–554; Angew. Chem. 2009, 121, 559–562.
- 5A. Falk, A.-L. Göderz, H. G. Schmalz, Angew. Chem. Int. Ed. 2013, 52, 1576–1580; Angew. Chem. 2013, 125, 1617–1621.
- 6These surrogates display toxicity similar to HCN:
- 6a“Cyanotrimethylsilane”: W. C. Groutas, in e-EROS Encyclopedia of Reagents for Organic Synthesis, Wiley, Chichester, 2001;
- 6b“Acetone cyanohydrin”: S. A. Haroutounian, in e-EROS Encyclopedia of Reagents for Organic Synthesis, Wiley, Chichester, 2001.
- 7B. Gaspar, E. M. Carreira, Angew. Chem. Int. Ed. 2007, 46, 4519–4522; Angew. Chem. 2007, 119, 4603–4606.
- 8
- 8aX. Fang, P. Yu, B. Morandi, Science 2016, 351, 832–836; a computational analysis of the reaction mechanism:
- 8bS.-F. Ni, T.-L. Yang, L. Dang, Organometallics 2017, 36, 2746–2754.
- 9Recent reviews of transition-metal-catalyzed transfer processes:
- 9aB. N. Bhawal, B. Morandi, Angew. Chem. Int. Ed. 2019, https://doi.org/10.1002/anie.201803797; Angew. Chem. 2019, https://doi.org/10.1002/ange.201803797;
- 9bB. N. Bhawal, B. Morandi, Chem. Eur. J. 2017, 23, 12004–12013;
- 9cB. N. Bhawal, B. Morandi, ACS Catal. 2016, 6, 7528–7535.
- 10A review: S. Keess, M. Oestreich, Chem. Sci. 2017, 8, 4688–4695.
- 11A comprehensive quantum-chemical analysis of various reaction pathways:
- 11aS. Banerjee, K. Vanka, ACS Catal. 2018, 8, 6163–6176; relevant experimental work suggesting competing reaction pathways:
- 11bI. Chatterjee, M. Oestreich, Org. Lett. 2016, 18, 2463–2466.
- 12A review of transfer hydrosilylation:
- 12aM. Oestreich, Angew. Chem. Int. Ed. 2016, 55, 494–499; Angew. Chem. 2016, 128, 504–509;
- 12bA. Simonneau, M. Oestreich, Angew. Chem. Int. Ed. 2013, 52, 11905–11907; Angew. Chem. 2013, 125, 12121–12124;
- 12cA. Simonneau, M. Oestreich, Nat. Chem. 2015, 7, 816–822; a computational analysis of the reaction mechanism:
- 12dK. Sakata, H. Fujimoto, Organometallics 2015, 34, 236–241.
- 13I. Chatterjee, Z.-W. Qu, S. Grimme, M. Oestreich, Angew. Chem. Int. Ed. 2015, 54, 12158–12162; Angew. Chem. 2015, 127, 12326–12330.
- 14S. Keess, M. Oestreich, Chem. Eur. J. 2017, 23, 5925–5928.
- 15Related transfer processes using cyclohexa-1,3-diene-based surrogates: W. Yuan, P. Orecchia, M. Oestreich, Chem. Commun. 2017, 53, 10390–10393.
- 16J. Li, Y. Okuda, J. Zhao, S. Mori, Y. Nishihara, Org. Lett. 2014, 16, 5220–5223.
- 17A. Bhunia, K. Bergander, A. Studer, J. Am. Chem. Soc. 2018, 140, 16353–16359.
- 18K. Ohkata, Y. Tamura, B. B. Shetuni, R. Takagi, W. Miyanaga, S. Kojima, L. A. Paquette, J. Am. Chem. Soc. 2004, 126, 16783–16792.
- 19
- 19aThe computed pathway of its formation: Ref. [13];
- 19band its subsequent formation in high yield: J. Möbus, T. vom Stein, D. W. Stephan, Chem. Commun. 2016, 52, 6387–6390.
- 20I. B. Sivaev, V. I. Bregadze, Coord. Chem. Rev. 2014, 270–271, 75–88.
- 21A. Sundararaman, F. A. Jäkle, J. Organomet. Chem. 2003, 681, 134–142.
- 22D. J. Parks, W. E. Piers, G. P. A. Yap, Organometallics 1998, 17, 5492–5503.
- 23A useful discussion of the 11B and 13C chemical shifts of three- and four-coordinate boron compounds with and without C≡N ligands: A. Bernsdorf, H. Brand, R. Hellmann, M. Köckerling, A. Schulz, A. Villinger, K. Voss, J. Am. Chem. Soc. 2009, 131, 8958–8970.
- 24D. S. Marynick, L. Throckmorton, R. Bacquet, J. Am. Chem. Soc. 1982, 104, 1–5.