Tryptamine Synthesis by Iron Porphyrin Catalyzed C−H Functionalization of Indoles with Diazoacetonitrile
Katharina J. Hock
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorAnja Knorrscheidt
Leibniz Institute of Plant Biochemistry, Weinberg 3, 06120 Halle (Saale), Germany
Search for more papers by this authorRenè Hommelsheim
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorDr. Junming Ho
School of Chemistry, University of New South Wales, Sydney, NSW, 2052 Australia
Search for more papers by this authorCorresponding Author
Jun.-Prof. Dr. Martin J. Weissenborn
Leibniz Institute of Plant Biochemistry, Weinberg 3, 06120 Halle (Saale), Germany
Institute of Chemistry, Martin-Luther-University Halle-Wittenberg, Kurt-Mothes-Str. 2, 06120 Halle (Saale), Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Rene M. Koenigs
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorKatharina J. Hock
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorAnja Knorrscheidt
Leibniz Institute of Plant Biochemistry, Weinberg 3, 06120 Halle (Saale), Germany
Search for more papers by this authorRenè Hommelsheim
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorDr. Junming Ho
School of Chemistry, University of New South Wales, Sydney, NSW, 2052 Australia
Search for more papers by this authorCorresponding Author
Jun.-Prof. Dr. Martin J. Weissenborn
Leibniz Institute of Plant Biochemistry, Weinberg 3, 06120 Halle (Saale), Germany
Institute of Chemistry, Martin-Luther-University Halle-Wittenberg, Kurt-Mothes-Str. 2, 06120 Halle (Saale), Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Rene M. Koenigs
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorGraphical Abstract
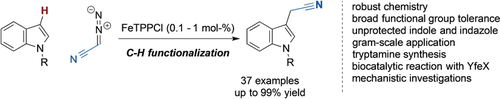
An iron porphyrin catalyst furnishes acetonitrile-functionalized indole derivatives by C−H functionalization reactions of protected and unprotected indole heterocycles with diazoacetonitrile. This process streamlines the synthesis of valuable tryptamines for applications in total synthesis and drug discovery.
Abstract
The functionalization of C−H bonds with non-precious metal catalysts is an important research area for the development of efficient and sustainable processes. Herein, we describe the development of iron porphyrin catalyzed reactions of diazoacetonitrile with N-heterocycles yielding important precursors of tryptamines, along with experimental mechanistic studies and proof-of-concept studies of an enzymatic process with YfeX enzyme. By using readily available FeTPPCl, we achieved the highly efficient C−H functionalization of indole and indazole heterocycles. These transformations feature mild reaction conditions, excellent yields with broad functional group tolerance, can be conducted on gram scale, and thus provide a unique streamlined access to tryptamines.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201813631-sup-0001-misc_information.pdf7.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected review articles on precious metal catalysts in C−H functionalization, see:
- 1aH. M. L. Davies, J. R. Manning, Nature 2008, 451, 417–424;
- 1bA. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire, M. A. McKervey, Chem. Rev. 2015, 115, 9981–10080;
- 1cM. P. Doyle, R. Duffy, M. Ratnikov, Z. Zhou, Chem. Rev. 2010, 110, 704–724;
- 1dH. M. L. Davies, D. Morton, Chem. Soc. Rev. 2011, 40, 1857–1869;
- 1eY. Xia, D. Qiu, J. Wang, Chem. Rev. 2017, 117, 13810–13889;
- 1fZ. Sheng, Z. Zhang, C. Chu, Y. Zhang, J. Wang, Tetrahedron 2017, 73, 4011–4022.
- 2For selected review articles on C−H activation, see:
- 2aS. A. Girard, T. Knauber, C.-J. Li, Angew. Chem. Int. Ed. 2014, 53, 74–100; Angew. Chem. 2014, 126, 76–103;
- 2bB. Li, P. H. Dixneuf, Chem. Soc. Rev. 2013, 42, 5744–5767;
- 2cT. Gensch, M. N. Hopkinson, F. Glorius, J. Wencel-Delord, Chem. Soc. Rev. 2016, 45, 2900–2936;
- 2dM. Moselage, J. Lie, L. Ackermann, ACS Catal. 2016, 6, 498–525.
- 3For selected review articles on iron catalysis, see:
- 3aH.-J. Knölker, I. Bauer, Chem. Rev. 2015, 115, 3170–3387;
- 3bS.-F. Zhu, Q.-L. Zhou, Natl. Sci. Rev. 2014, 1, 580–603;
- 3cR. Shang, L. Ilies, E. Nakamura, Chem. Rev. 2017, 117, 9086–9139;
- 3dC. Bolm, J. Legros, J. Le Paih, L. Zani, Chem. Rev. 2004, 104, 6217–6254;
- 3eB. Plietker, Iron Catalysis in Organic Chemistry: Reactions and applications, 2nd ed., Wiley-VCH, Weinheim, 2008.
10.1002/9783527623273 Google Scholar
- 4For selected review articles on iron porphyrin complexes in catalysis, see:
- 4aO. F. Brandenberg, R. Fasan, F. H. Arnold, Curr. Opin. Biotechnol. 2017, 47, 102–111;
- 4bS. Sahu, D. P. Goldberg, J. Am. Chem. Soc. 2016, 138, 11410–11428;
- 4cL. Que Jr, W. B. Tolman, Nature 2008, 455, 333–340;
- 4dE. I. Solomon, A. Decker, N. Lehnert, Proc. Natl. Acad. Sci. USA 2003, 100, 3589–3594.
- 5For selected articles on carbene transfer reactions with iron catalysts, see:
- 5aB. Morandi, E. M. Carreira, Angew. Chem. Int. Ed. 2010, 49, 938–941; Angew. Chem. 2010, 122, 950–953;
- 5bM. S. Holzwarth, I. Alt, B. Plietker, Angew. Chem. Int. Ed. 2012, 51, 5351–5354; Angew. Chem. 2012, 124, 5447–5450;
- 5cJ. Day, B. McKeever-Abbas, J. Dowden, Angew. Chem. Int. Ed. 2016, 55, 5809–5813; Angew. Chem. 2016, 128, 5903–5907;
- 5dJ. R. Griffin, C. I. Wendell, J. A. Garwin, M. C. White, J. Am. Chem. Soc. 2017, 139, 13624–13627;
- 5eD. M. Carminati, D. Intrieri, A. Caselli, S. Le Gac, B. Boitrel, L. Toma, L. Legnani, E. Gallo, Chem. Eur. J. 2016, 22, 13599–13612;
- 5fC. Empel, K. J. Hock, R. M. Koenigs, Chem. Commun. 2019, 55, 338–341.
- 6For selected articles on enzymatic carbene and nitrene transfer reactions, see:
- 6aP. S. Coelho, E. M. Brustad, A. Kannak, F. H. Arnold, Science 2013, 339, 307–310;
- 6bK. Chen, X. Huang, S. B. J. Kan, R. K. Zhang, F. H. Arnold, Science 2018, 360, 71–75;
- 6cT. Hayashi, M. Tinzl, T. Mori, U. Krengel, J. Poope, J. Soelbeer, D. Klose, G. Jeschke, M. Reiher, D. Hilvert, Nat. Catal. 2018, 1, 578–584;
- 6dM. J. Weissenborn, S. A. Low, N. Borlinghaus, M. Kuhn, S. Kummer, F. Rami, B. Plietker, B. Hauer, ChemCatChem 2016, 8, 1636–1640;
- 6eG. Sreenilayam, E. J. Moore, V. Steck, R. Fasan, Adv. Synth. Catal. 2017, 359, 2076–2089;
- 6fP. Bajaj, G. Sreenilayam, V. Tyagi, R. Fasan, Angew. Chem. Int. Ed. 2016, 55, 16110–16114; Angew. Chem. 2016, 128, 16344–16348; during the preparation of this manuscript, Fasan and co-workers reported on a similar reaction with ethyl diazoacetate and an enzymatic reaction of diazoacetonitrile; see:
- 6gD. A. Vargas, A. Tinoco, V. Tyagi, R. Fasan, Angew. Chem. Int. Ed. 2018, 57, 9911–9915; Angew. Chem. 2018, 130, 10059–10063;
- 6hA. L. Chandgude, R. Fasan, Angew. Chem. Int. Ed. 2018, 57, 15852–15856; Angew. Chem. 2018, 130, 16078–16082.
- 7
- 7aT. Curtius, Ber. Dtsch. Chem. Ges. 1898, 31, 2489–2492;
- 7bD. D. Phillips, W. C. Champion, J. Am. Chem. Soc. 1956, 78, 5452.
- 8For selected articles on the carbene reactivity of diazoacetonitrile, see:
- 8aK. J. Hock, R. Spitzner, R. M. Koenigs, Green Chem. 2017, 19, 2118–2122;
- 8bK. J. Hock, L. Mertens, R. Hommelsheim, R. Spitzner, R. M. Koenigs, Chem. Commun. 2017, 53, 6577–6580;
- 8cC. Empel, K. J. Hock, R. M. Koenigs, Org. Biomol. Chem. 2018, 16, 7129–7133;
- 8dC. V. Galliford, K. A. Scheidt, J. Org. Chem. 2007, 72, 1811–1813;
- 8eY. Ferrand, P. Le Maux, G. Simmoneaux, Tetrahedron: Asymmetry 2005, 16, 3829–3836;
- 8fF.-X. Felpin, E. Doris, A. Wagner, A. Valleix, B. Rousseau, C. Mioskowski, J. Org. Chem. 2001, 66, 305–308.
- 9
- 9aS. Takano, T. Nishimura, K. Ogasawawa, Heterocycles 1977, 6, 1167–1171;
- 9bM. Eckstein, S. Misztal, A. Terczynska, M. Adamus, PL130769, 1984;
- 9cL. T. Pierce, M. M. Cahill, F. O. McCarthy, Tetrahedron 2011, 67, 4601–4611;
- 9dA. Tsotinis, M. Vlachou, D. P. Papahatjis, T. Calogeropoulou, S. P. Nikas, P. J. Garratt, V. Piccio, S. Vonhoff, K. Davidson, M.-T. Teh, D. Sugden, J. Med. Chem. 2006, 49, 3509–3519.
- 10E. Wenkert, M. E. Alonso, H. E. Gottlieb, E. L. Sanchez, R. Pellicciari, P. Cogolli, J. Org. Chem. 1977, 42, 3945–3949.
- 11A. H. Sandtorv, Adv. Synth. Catal. 2015, 357, 2403–2435.
- 12
- 12aM. Delgado-Rebollo, A. Prieto, P. J. Pérez, ChemCatChem 2014, 6, 2047–2052;
- 12bM. Sarkar, P. Daw, T. Ghatak, J. K. Bera, Chem. Eur. J. 2014, 20, 16537–16549;
- 12cG. Özüduru, T. Schubach, M. M. Boyson, Org. Lett. 2012, 14, 4990–4993;
- 12dF. Gnad, M. Poleschak, O. Reiser, Tetrahedron Lett. 2004, 45, 4277–4280.
- 13For iron-catalyzed reactions with donor–acceptor diazoalkanes, see: Y. Cai, S.-F. Zhu, G.-P. Wang, Q.-L. Zhou, Adv. Synth. Catal. 2011, 353, 2939–2944.
- 14L. K. Baumann, H. M. Mbuvi, G. Du, L. K. Woo, Organometallics 2007, 26, 3995–4002.
- 15Diazoacetonitrile was prepared in continuous flow by mixing aqueous solutions of aminoacetonitrile hydrochloride and sodium nitrite (microreactor: Little Things Factory MR Lab MX, tubing 0.8 mm ID, back pressure regulator 20 psi) at 55 °C, residence time 1 min. The outlet was added into a consecutive batch transformation containing 1-methylindole and the respective metal catalyst. NMR studies revealed quantitative formation of diazoacetonitrile.
- 16TPP=meso-tetraphenylporphyrin.
- 17For details see the Supporting Information.
- 18
- 18aA. J. Cooke, D. Pitts, A. Johnson, D. C. Beshore, D. Hurzy, H. Mitchell, M. Fraley, C. McComas, K. Schirripa, S. P. Mercer, K. Nanda, D. Meng, J. Wu, K. Babaoglu, C.-S. Li, Q. Mao, Z. Qi, 2016, WO2016054807;
- 18bA. M. Winter-Vann, R. A. Baron, W. Wong, J. de la Cruz, J. D. York, D. M. Gooden, M. O. Bergo, S. G. Young, E. J. Toone, P. J. Casey, Proc. Natl. Acad. Sci. USA 2005, 102, 4336–4341;
- 18cM.-L. Go, J. L. Leow, S. K. Gorla, A. P. Schüller, M. Wang, P. J. Casey, J. Med. Chem. 2010, 53, 6838–6850;
- 18dK. Fukunaga, F. Han, N. Shioda, S. Moriguchi, J. Kasahara, Y. Shirasaki, Cardiovasc. Ther. 2006, 24, 88–100.
- 19X. Liu, Z. Yuan, J. Wang, Y. Cui, S. Liu, Y. Ma, L. Gu, S. Xu, Biochem. Biophys. Res. Commun. 2017, 484, 40–44.
- 20In the background reactions we observed an erosion of the deuterium label of the starting material of 65 % and 79 %, which shows that deuteron-1 undergoes D/H exchange in the reaction medium. This observation might explain the reduced deuterium content of the reaction product 3 a.
- 21
- 21aC. Kong, N. Jana, C. Jones, T. Driver, J. Am. Chem. Soc. 2016, 138, 13271–13280;
- 21bR. Singh, J. N. Kolev, P. A. Sutera, R. Fasan, ACS Catal. 2015, 5, 1685–1691.
- 22T. Brückl, R. D. Baxter, Y. Ishihara, P. S. Baran, Acc. Chem. Res. 2012, 45, 826–839.