Aluminum(I)/Boron(III) Redox Reactions
Alexander Hofmann
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorDr. Conor Pranckevicius
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorTobias Tröster
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Holger Braunschweig
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorAlexander Hofmann
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorDr. Conor Pranckevicius
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorTobias Tröster
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Holger Braunschweig
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorGraphical Abstract
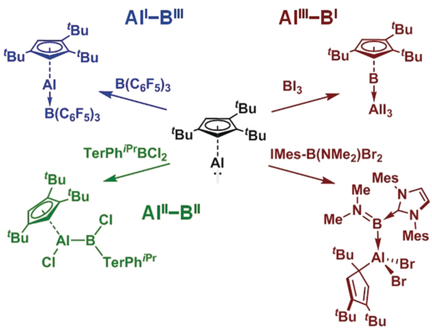
All good things come in threes: Reactions of an AlI nucleophile with BIII species leads to three distinct modes of reactivity: adduct formation (AlI-BIII), oxidative addition (AlII-BII), and reduction (AlIII-BI). IMes=1,3-dimesitylimidazol-2-ylidene, TerPhiPr=bis-2,6-(2,4,6-triisopropylphenyl)phenyl.
Abstract
Reactions between BIII species and the novel nucleophilic cyclopentadienyl-stabilized AlI reagent (1) result in a diversity of complexes bearing different Al/B oxidation states and coordination geometries. With the triarylborane B(C6F5)3, a simple AlI→BIII adduct is formed. In contrast, a bulky aryldihaloborane undergoes oxidative addition with the formation of a covalent bora-alane species. With an N-heterocyclic carbene-stabilized amino(bromo)borenium ion, a redox reaction was observed, where the product is a borylene-alane BI→AlIII complex. Additionally, reaction of 1 with BI3 results in complete scrambling of all of the Al/B-bound substituents, and the formation of a cyclopentadienylboron(I)→AlI3 complex. These latter reactions are the first examples of the reduction of a boron(III) compound to a borylene by a p-block reagent, and illustrate how subtle changes in the nature of the borane can result in highly divergent reaction outcomes.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201813619-sup-0001-misc_information.pdf1.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aH. Braunschweig, R. D. Dewhurst, V. H. Gessner, Chem. Soc. Rev. 2013, 42, 3197–3208;
- 1bM. Soleilhavoup, G. Bertrand, Angew. Chem. Int. Ed. 2017, 56, 10282–10292; Angew. Chem. 2017, 129, 10416–10426;
- 1cM.-A. Légaré, G. Bélanger-Chabot, R. D. Dewhurst, E. Welz, I. Krummenacher, B. Engels, H. Braunschweig, Science 2018, 359, 896–900;
- 1dM. Arrowsmith, H. Braunschweig, T. E. Stennett, Angew. Chem. Int. Ed. 2017, 56, 96–115; Angew. Chem. 2017, 129, 100–120;
- 1eF. Dahcheh, D. Martin, D. W. Stephan, G. Bertrand, Angew. Chem. Int. Ed. 2014, 53, 13159–13163; Angew. Chem. 2014, 126, 13375–13379;
- 1fC. Pranckevicius, J. O. C. Jimenéz-Halla, M. Kirsch, I. Krummenacher, H. Braunschweig, J. Am. Chem. Soc. 2018, 140, 10524–10529.
- 2
- 2a“The Chemistry of Low-Valent Organoaluminum Species”: R. J. Wehmschulte in PATAI′S Chemistry of Functional Groups (Ed.: ), Wiley, Hoboken, 2016;
- 2bH. W. Roesky, S. S. Kumar, Chem. Commun. 2005, 4027–4038;
- 2cW. Uhl in Reviews in Inorganic Chemistry, Vol. 18, deGruyter, Berlin, 1998, S. 239;
- 2dM. Mocker, C. Robl, H. Schnöckel, Angew. Chem. Int. Ed. Engl. 1994, 33, 1754–1755; Angew. Chem. 1994, 106, 1860–1861;
- 2eC. Schnitter, H. W. Roesky, C. Röpken, R. Herbst-Irmer, H.-G. Schmidt, M. Noltemeyer, Angew. Chem. Int. Ed. 1998, 37, 1952–1955;
10.1002/(SICI)1521-3773(19980803)37:13/14<1952::AID-ANIE1952>3.0.CO;2-U CAS Web of Science® Google ScholarAngew. Chem. 1998, 110, 2059–2062;10.1002/(SICI)1521-3757(19980703)110:13/14<2059::AID-ANGE2059>3.0.CO;2-F Web of Science® Google Scholar
- 2fM. Driess, H. Grützmacher, Angew. Chem. Int. Ed. Engl. 1996, 35, 828–856; Angew. Chem. 1996, 108, 900–929;
- 2gC. Dohmeier, D. Loos, H. Schnöckel, Angew. Chem. Int. Ed. Engl. 1996, 35, 129–149; Angew. Chem. 1996, 108, 141–161;
- 2hC. Dohmeier, E. Baum, A. Ecker, R. Köppe, H. Schnöckel, Organometallics 1996, 15, 4702–4706;
- 2iH. Sitzmann, M. F. Lappert, C. Dohmeier, C. Üffing, H. Schnöckel, J. Organomet. Chem. 1998, 561, 203–208;
- 2jM. Huber, H. Schnöckel, Inorg. Chim. Acta 2008, 361, 457–461;
- 2kW. W. Tomlinson, D. H. Mayo, R. M. Wilson, J. P. Hooper, J. Phys. Chem. A 2017, 121, 4678–4687.
- 3C. Dohmeier, C. Robl, M. Tacke, H. Schnöckel, Angew. Chem. Int. Ed. Engl. 1991, 30, 564–565; Angew. Chem. 1991, 103, 594–595.
- 4
- 4aS. Schulz, L. Häming, R. Herbst-Irmer, H. W. Roesky, G. M. Sheldrick, Angew. Chem. Int. Ed. Engl. 1994, 33, 969–970; Angew. Chem. 1994, 106, 1052–1054;
- 4bS. Schulz, H. W. Roesky, H. J. Koch, G. M. Sheldrick, D. Stalke, A. Kuhn, Angew. Chem. Int. Ed. Engl. 1993, 32, 1729–1731; Angew. Chem. 1993, 105, 1828–1830;
- 4cA. C. Stelzer, P. Hrobárik, T. Braun, M. Kaupp, B. Braun-Cula, Inorg. Chem. 2016, 55, 4915–4923.
- 5
- 5aJ. Weßing, C. Göbel, B. Weber, C. Gemel, R. A. Fischer, Inorg. Chem. 2017, 56, 3517–3525;
- 5bM. Molon, C. Gemel, R. A. Fischer, J. Organomet. Chem. 2014, 751, 573–578;
- 5cM. Molon, C. Gemel, R. A. Fischer, Eur. J. Inorg. Chem. 2013, 3616–3622;
- 5dP. W. Roesky, Dalton Trans. 2009, 1887–1893;
- 5eM. T. Gamer, P. W. Roesky, S. N. Konchenko, P. Nava, R. Ahlrichs, Angew. Chem. Int. Ed. 2006, 45, 4447–4451; Angew. Chem. 2006, 118, 4558–4561;
- 5fB. Buchin, T. Steinke, C. Gemel, T. Cadenbach, R. A. Fischer, Z. Anorg. Allg. Chem. 2005, 631, 2756–2762;
- 5gT. Steinke, C. Gemel, M. Cokoja, M. Winter, R. A. Fischer, Angew. Chem. Int. Ed. 2004, 43, 2299–2302; Angew. Chem. 2004, 116, 2349–2352;
- 5hC. Dohmeier, H. Krautscheid, H. Schnöckel, Angew. Chem. Int. Ed. Engl. 1995, 33, 2482–2483;
- 5iR. A. Fischer, J. Weiß, Angew. Chem. Int. Ed. 1999, 38, 2830–2850;
10.1002/(SICI)1521-3773(19991004)38:19<2830::AID-ANIE2830>3.0.CO;2-E CAS PubMed Web of Science® Google ScholarAngew. Chem. 1999, 111, 3002–3022;10.1002/(SICI)1521-3757(19991004)111:19<3002::AID-ANGE3002>3.0.CO;2-B Web of Science® Google Scholar
- 5jJ. Weiss, D. Stetzkamp, B. Nuber, R. A. Fischer, C. Boehme, G. Frenking, Angew. Chem. Int. Ed. Engl. 1997, 36, 70–72; Angew. Chem. 1997, 109, 95–97;
- 5kJ. D. Gorden, C. L. B. Macdonald, A. H. Cowley, Chem. Commun. 2001, 75–76.
- 6C. Cui, H. W. Roesky, H.-G. Schmidt, M. Noltemeyer, H. Hao, F. Cimpoesu, Angew. Chem. Int. Ed. 2000, 39, 4274–4276;
10.1002/1521-3773(20001201)39:23<4274::AID-ANIE4274>3.0.CO;2-K CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 4444–4446.
- 7
- 7aC. Ganesamoorthy, D. Bläser, C. Wölper, S. Schulz, Chem. Commun. 2014, 50, 12382–12384;
- 7bC. Cui, S. Köpke, R. Herbst-Irmer, H. W. Roesky, M. Noltemeyer, H.-G. Schmidt, B. Wrackmeyer, J. Am. Chem. Soc. 2001, 123, 9091–9098;
- 7cH. Zhu, J. Chai, H. Fan, H. W. Roesky, U. N. Nehete, H.-G. Schmidt, M. Noltemeyer, Eur. J. Inorg. Chem. 2005, 2147–2150;
- 7dH. Zhu, J. Chai, H. Fan, H. W. Roesky, C. He, V. Jancik, H.-G. Schmidt, M. Noltemeyer, W. A. Merrill, P. P. Power, Angew. Chem. Int. Ed. 2005, 44, 5090–5093; Angew. Chem. 2005, 117, 5220–5223;
- 7eH. Zhu, R. B. Oswald, H. Fan, H. W. Roesky, Q. Ma, Z. Yang, H.-G. Schmidt, M. Noltemeyer, K. Starke, N. S. Hosmane, J. Am. Chem. Soc. 2006, 128, 5100–5108;
- 7fT. Chu, I. Korobkov, G. I. Nikonov, J. Am. Chem. Soc. 2014, 136, 9195–9202;
- 7gC. Ganesamoorthy, D. Bläser, C. Wölper, S. Schulz, Angew. Chem. Int. Ed. 2014, 53, 11587–11591; Angew. Chem. 2014, 126, 11771–11775;
- 7hM. R. Crimmin, M. J. Butler, A. J. P. White, Chem. Commun. 2015, 51, 15994–15996;
- 7iT. Chu, Y. Boyko, I. Korobkov, G. I. Nikonov, Organometallics 2015, 34, 5363–5365;
- 7jL. Kong, R. Ganguly, Y. Li, R. Kinjo, Chem. Eur. J. 2016, 22, 1922–1925;
- 7kX. Zhang, Z. Cao, Dalton Trans. 2016, 45, 10355–10365;
- 7lT. Chu, Y. Boyko, I. Korobkov, L. G. Kuzmina, J. A. K. Howard, G. I. Nikonov, Inorg. Chem. 2016, 55, 9099–9104;
- 7mW. W. Schoeller, G. D. Frey, Inorg. Chem. 2016, 55, 10947–10954;
- 7nB. Li, S. Kundu, H. Zhu, H. Keil, R. Herbst-Irmer, D. Stalke, G. Frenking, D. M. Andrada, H. W. Roesky, Chem. Commun. 2017, 53, 2543–2546;
- 7oL. Tuscher, C. Helling, C. Wölper, W. Frank, A. S. Nizovtsev, S. Schulz, Chem. Eur. J. 2018, 24, 3241–3250;
- 7pC. Bakewell, A. J. P. White, M. R. Crimmin, Angew. Chem. Int. Ed. 2018, 57, 6638–6642; Angew. Chem. 2018, 130, 6748–6752.
- 8
- 8aC. Cui, H. W. Roesky, H.-G. Schmidt, M. Noltemeyer, Angew. Chem. Int. Ed. 2000, 39, 4531–4533;
10.1002/1521-3773(20001215)39:24<4531::AID-ANIE4531>3.0.CO;2-K CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 4705–4707;
- 8bN. J. Hardman, C. Cui, H. W. Roesky, W. H. Fink, P. P. Power, Angew. Chem. Int. Ed. 2001, 40, 2172–2174;
10.1002/1521-3773(20010601)40:11<2172::AID-ANIE2172>3.0.CO;2-Y CAS PubMed Web of Science® Google ScholarAngew. Chem. 2001, 113, 2230–2232;
- 8cH. Zhu, J. Chai, V. Chandrasekhar, H. W. Roesky, J. Magull, D. Vidovic, H.-G. Schmidt, M. Noltemeyer, P. P. Power, W. A. Merrill, J. Am. Chem. Soc. 2004, 126, 9472–9473;
- 8dY. Peng, H. Fan, H. Zhu, H. W. Roesky, J. Magull, C. E. Hughes, Angew. Chem. Int. Ed. 2004, 43, 3443–3445; Angew. Chem. 2004, 116, 3525–3527;
- 8eH. Zhu, J. Chai, A. Stasch, H. W. Roesky, T. Blunck, D. Vidovic, J. Magull, H.-G. Schmidt, M. Noltemeyer, Eur. J. Inorg. Chem. 2004, 4046–4051;
- 8fY. Peng, H. Fan, V. Jancik, H. W. Roesky, R. Herbst-Irmer, Angew. Chem. Int. Ed. 2004, 43, 6190–6192; Angew. Chem. 2004, 116, 6316–6318;
- 8gH. Zhu, J. Chai, V. Jancik, H. W. Roesky, W. A. Merrill, P. P. Power, J. Am. Chem. Soc. 2005, 127, 10170–10171;
- 8hH. Zhu, Z. Yang, J. Magull, H. W. Roesky, H.-G. Schmidt, M. Noltemeyer, Organometallics 2005, 24, 6420–6425;
- 8iZ. Yang, X. Ma, R. B. Oswald, H. W. Roesky, M. Noltemeyer, J. Am. Chem. Soc. 2006, 128, 12406–12407;
- 8jT. Chu, S. F. Vyboishchikov, B. Gabidullin, G. I. Nikonov, Angew. Chem. Int. Ed. 2016, 55, 13306–13311; Angew. Chem. 2016, 128, 13500–13505;
- 8kT. Chu, S. F. Vyboishchikov, B. M. Gabidullin, G. I. Nikonov, Inorg. Chem. 2017, 56, 5993–5997;
- 8lT. Chu, S. F. Vyboishchikov, B. M. Gabidullin, G. I. Nikonov, J. Am. Chem. Soc. 2017, 139, 8804–8807;
- 8mS. Sinhababu, S. Kundu, A. N. Paesch, R. Herbst-Irmer, D. Stalke, H. W. Roesky, Eur. J. Inorg. Chem. 2018, 2237–2240.
- 9
- 9aA. Kempter, C. Gemel, R. A. Fischer, Chem. Commun. 2006, 1551–1553;
- 9bA. Kempter, C. Gemel, R. A. Fischer, Chem. Eur. J. 2007, 13, 2990–3000.
- 10P. Bag, A. Porzelt, P. J. Altmann, S. Inoue, J. Am. Chem. Soc. 2017, 139, 14384–14387.
- 11J. Hicks, P. Vasko, J. M. Goicoechea, S. Aldridge, Nature 2018, 557, 92–95.
- 12A. Hofmann, T. Tröster, T. Kupfer, H. Braunschweig, Chem. Sci. 2019, https://doi.org/10.1039/C8SC05175E.
- 13
- 13aJ. D. Gorden, A. Voigt, C. L. B. Macdonald, J. S. Silverman, A. H. Cowley, J. Am. Chem. Soc. 2000, 122, 950–951;
- 13bP. E. Romero, W. E. Piers, S. A. Decker, D. Chau, T. K. Woo, M. Parvez, Organometallics 2003, 22, 1266–1274.
- 14
- 14aN. Dettenrieder, H. M. Dietrich, C. Schädle, C. Maichle-Mössmer, K. W. Törnroos, R. Anwander, Angew. Chem. Int. Ed. 2012, 51, 4461–4465; Angew. Chem. 2012, 124, 4537–4541;
- 14bN. Dettenrieder, C. O. Hollfelder, L. N. Jende, C. Maichle-Mössmer, R. Anwander, Organometallics 2014, 33, 1528–1531;
- 14cN. Dettenrieder, C. Schädle, C. Maichle-Mössmer, R. Anwander, Dalton Trans. 2014, 43, 15760–15770;
- 14dD. Dange, C. P. Sindlinger, S. Aldridge, C. Jones, Chem. Commun. 2017, 53, 149–152;
- 14eA. V. Protchenko, J. Urbano, J. A. B. Abdalla, J. Campos, D. Vidovic, A. D. Schwarz, M. P. Blake, P. Mountford, C. Jones, S. Aldridge, Angew. Chem. Int. Ed. 2017, 56, 15098–15102; Angew. Chem. 2017, 129, 15294–15298.
- 15A. Hofmann, A. Lamprecht, J. O. C. Jiménez-Halla, T. Tröster, R. D. Dewhurst, C. Lenczyk, H. Braunschweig, Chem. Eur. J. 2018, 24, 11795–11802.
- 16A. Y. Timoshkin, G. Frenking, J. Am. Chem. Soc. 2002, 124, 7240–7248.
- 17
- 17aL. Kong, W. Lu, L. Yongxin, R. Ganguly, R. Kinjo, Inorg. Chem. 2017, 56, 5586–5593;
- 17bH. Braunschweig, R. D. Dewhurst, L. Pentecost, K. Radacki, A. Vargas, Q. Ye, Angew. Chem. Int. Ed. 2016, 55, 436–440; Angew. Chem. 2016, 128, 447–451.
- 18
- 18aU. Holtmann, P. Jutzi, T. Kühler, B. Neumann, H.-G. Stammler, Organometallics 1999, 18, 5531–5538;
- 18bP. Greiwe, A. Bethäuser, H. Pritzkow, T. Kühler, P. Jutzi, W. Siebert, Eur. J. Inorg. Chem. 2000, 1927–1929.
- 19Y. Dang, L. Meng, M. Qin, Q. Li, X. Li, J. Mol. Model. 2017, 24, 7.
- 20
- 20aA. H. Cowley, V. Lomelí, A. Voigt, J. Am. Chem. Soc. 1998, 120, 6401–6402;
- 20bC. L. B. Macdonald, A. H. Cowley, J. Am. Chem. Soc. 1999, 121, 12113–12126;
- 20cJ. Uddin, C. Boehme, G. Frenking, Organometallics 2000, 19, 571–582;
- 20dD. Vidovic, M. Findlater, G. Reeske, A. H. Cowley, Chem. Commun. 2006, 3786–3787.