Donor Rhodium Carbenes by Retro-Buchner Reaction
Mauro Mato
Institute of Chemical Research of Catalonia (ICIQ), Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
Departament de Química Analítica i Química Orgànica, Universitat Rovira i Virgili, C/ Marcel⋅li Domingo s/n, 43007 Tarragona, Spain
Search for more papers by this authorCorresponding Author
Prof. Antonio M. Echavarren
Institute of Chemical Research of Catalonia (ICIQ), Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
Departament de Química Analítica i Química Orgànica, Universitat Rovira i Virgili, C/ Marcel⋅li Domingo s/n, 43007 Tarragona, Spain
Search for more papers by this authorMauro Mato
Institute of Chemical Research of Catalonia (ICIQ), Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
Departament de Química Analítica i Química Orgànica, Universitat Rovira i Virgili, C/ Marcel⋅li Domingo s/n, 43007 Tarragona, Spain
Search for more papers by this authorCorresponding Author
Prof. Antonio M. Echavarren
Institute of Chemical Research of Catalonia (ICIQ), Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
Departament de Química Analítica i Química Orgànica, Universitat Rovira i Virgili, C/ Marcel⋅li Domingo s/n, 43007 Tarragona, Spain
Search for more papers by this authorGraphical Abstract
Abstract
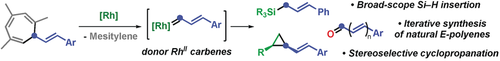
Rhodium carbenes are key intermediates in a range of cycloadditions and insertion reactions. Herein, we report the first generation of donor RhII carbenes by decarbenation of 7-substituted 1,3,5-cycloheptatrienes. This discovery unlocks an improved retro-Buchner-cyclopropanation sequence, a Si−H insertion reaction for a broad-scope synthesis of allylsilanes, and a new method for the vinylogation of aldehydes. The last strategy led to the development of an iterative synthesis of E-polyenes, and to the total synthesis of navenones B and C.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201813512-sup-0001-misc_information.pdf15.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. de Meijere, T.-J. Schulz, R. R. Kostikov, F. Graupner, T. Murr, T. Bielfeldt, Synthesis 1991, 547–560;
- 1bV. K. Singh, A. DattaGupta, G. Sekar, Synthesis 1997, 137–148;
- 1cH. M. L. Davies, P. R. Bruzinski, D. H. Lake, N. Kong, M. J. Fall, J. Am. Chem. Soc. 1996, 118, 6897–6907;
- 1dM. P. Doyle, M. A. McKervey, T. Ye in Modern Catalytic Methods for Organic Synthesis with Diazo Compounds: From Cyclopropanes to Ylides, Wiley, New York, 1998, pp. 163–220;
- 1e“Intermolecular Metal-Catalyzed Carbenoid Cyclopropanations”: H. M. L. Davies, E. G. Antoulinakis, Org. React. 2001, 57, 1;
- 1fH. Wang, D. M. Guptill, A. Varela-Alvarez, D. G. Musaev, H. M. L. Davies, Chem. Sci. 2013, 4, 2844–2850.
- 2
- 2aM. P. Doyle, D. C. Forbes, Chem. Rev. 1998, 98, 911–936;
- 2bH. M. L. Davies, J. R. Manning, Nature 2008, 451, 417–424;
- 2cM. P. Doyle, R. Duffy, M. Ratnikov, L. Zhou, Chem. Rev. 2010, 110, 704–724;
- 2dH. M. L. Davies, D. Morton, Chem. Soc. Rev. 2011, 40, 1857–1869;
- 2eK. Liao, S. Negretti, D. G. Musaev, J. Bacsa, H. M. L. Davies, Nature 2016, 533, 230–234;
- 2fK. Liao, T. C. Pickel, V. Boyarskikh, J. Bacsa, D. G. Musaev, H. M. L. Davies, Nature 2017, 551, 609–613.
- 3
- 3aS.-H. Lee, B. Clapham, G. Koch, J. Zimmermann, K. D. Janda, Org. Lett. 2003, 5, 511–514.
- 4
- 4aA. DeAngelis, M. T. Taylor, J. M. Fox, J. Am. Chem. Soc. 2009, 131, 1101–1105;
- 4bY. Xia, D. Qiu, J. Wang, Chem. Rev. 2017, 117, 13810–13889.
- 5
- 5aV. K. Aggarwal, E. Alonso, G. Hynd, K. M. Lydon, M. J. Palmer, M. Porcelloni, J. R. Studley, Angew. Chem. Int. Ed. 2001, 40, 1430–1433;
10.1002/1521-3773(20010417)40:8<1430::AID-ANIE1430>3.0.CO;2-W CAS PubMed Web of Science® Google ScholarAngew. Chem. 2001, 113, 1479–1482;
- 5bV. K. Aggarwal, J. de Vicente, R. V. Bonnert, Org. Lett. 2001, 3, 2785–2788;
- 5cR. P. Wurz, A. B. Charette, Org. Lett. 2002, 4, 4531–4533;
- 5dB. Morandi, E. M. Carreira, Science 2012, 335, 1471–1474.
- 6
- 6aK. P. Kornecki, J. F. Briones, V. Boyarskikh, F. Fullilove, J. Autschbach, K. E. Schrote, K. M. Lancaster, H. M. L. Davies, J. F. Berry, Science 2013, 342, 351–354;
- 6bC. Werlé, R. Goddard, A. Fürstner, Angew. Chem. Int. Ed. 2015, 54, 15452–15456; Angew. Chem. 2015, 127, 15672–15676.
- 7
- 7aH. M. L. Davies, R. E. J. Beckwith, Chem. Rev. 2003, 103, 2861–2904;
- 7bD. Zhu, J. Ma, K. Luo, H. Fu, L. Zhang, S. Zhu, Angew. Chem. Int. Ed. 2016, 55, 8452–8456; Angew. Chem. 2016, 128, 8592–8596.
- 8
- 8aT. Sammakia in Encyclopedia of Reagents for Organic Synthesis, Vol. 4 (Ed.: ), Wiley, Chichester, 1995, p. 1512;
- 8bV. K. Aggarwal, E. Alonso, I. Bae, G. Hynd, K. M. Lydon, M. J. Palmer, M. Patel, M. Porcelloni, J. Richardson, R. A. Stenson, J. R. Studley, J.-L. Vasse, C. L. Winn, J. Am. Chem. Soc. 2003, 125, 10926–10940;
- 8cJ. R. Fulton, V. K. Aggarwal, J. de Vicente, Eur. J. Org. Chem. 2005, 1479–1492.
- 9M. P. Doyle, J. H. Griffin, V. Bagheri, R. L. Dorow, Organometallics 1984, 3, 53–61.
- 10Reviews on the generation of RhII carbenes:
- 10aM. Jia, S. Ma, Angew. Chem. Int. Ed. 2016, 55, 9134–9166; Angew. Chem. 2016, 128, 9280–9313; from triazoles:
- 10bS. Chuprakov, S. W. Kwok, L. Zhang, L. Lercher, V. V. Fokin, J. Am. Chem. Soc. 2009, 131, 18034–18035;
- 10cY. Shi, A. V. Gulevich, V. Gevorgyan, Angew. Chem. Int. Ed. 2014, 53, 14191–14195; Angew. Chem. 2014, 126, 14415–14419;
- 10dT. Miura, T. Nakamuro, C.-J. Liang, M. Murakami, J. Am. Chem. Soc. 2014, 136, 15905–15908;
- 10eY. Chen, S. Dong, X. Xu, X. Liu, X. Feng, Angew. Chem. Int. Ed. 2018, 57, 16554–16558; Angew. Chem. 2018, 130, 16792–16796; from phenyliodonium ylides:
- 10fB. Moreau, A. B. Charette, J. Am. Chem. Soc. 2005, 127, 18014–18015; from cyclopropenes:
- 10gA. Archambeau, F. Miege, C. Meyer, J. Cossy, Angew. Chem. Int. Ed. 2012, 51, 11540–11544; Angew. Chem. 2012, 124, 11708–11712.
- 11
- 11aC. R. Solorio-Alvarado, A. M. Echavarren, J. Am. Chem. Soc. 2010, 132, 11881–11883; for a review, see:
- 11bM. Mato, C. García-Morales, A. M. Echavarren, ChemCatChem 2018, https://doi.org/10.1002/cctc.201801201.
- 12
- 12aC. R. Solorio-Alvarado, Y. Wang, A. M. Echavarren, J. Am. Chem. Soc. 2011, 133, 11952–11955;
- 12bB. Herlé, P. M. Holstein, A. M. Echavarren, ACS Catal. 2017, 7, 3668–3675.
- 13
- 13aY. Wang, P. R. McGonigal, B. Herlé, M. Besora, A. M. Echavarren, J. Am. Chem. Soc. 2014, 136, 801–809;
- 13bY. Wang, M. E. Muratore, Z. Rhong, A. M. Echavarren, Angew. Chem. Int. Ed. 2014, 53, 14022–14026; Angew. Chem. 2014, 126, 14246–14250;
- 13cX. Yin, M. Mato, A. M. Echavarren, Angew. Chem. Int. Ed. 2017, 56, 14591–14595; Angew. Chem. 2017, 129, 14783–14787.
- 14M. Mato, B. Herlé, A. M. Echavarren, Org. Lett. 2018, 20, 4341–4345.
- 15H. C. Volger, H. Hogeveen, C. F. Roobeek, Recl. Trav. Chim. Pays-Bas 1973, 92, 1223–1231.
- 16For reviews on cyclopropanation, see:
- 16aH. Lebel, J.-F. Marcoux, C. Molinaro, A. B. Charette, Chem. Rev. 2003, 103, 977–1050;
- 16bC. Ebner, E. M. Carreira, Chem. Rev. 2017, 117, 11651–11679.
- 17For Si−H insertions with Rh carbenes, see:
- 17aY. Landais, D. Planchenault, V. Weber, Tetrahedron Lett. 1994, 35, 9549–9552;
- 17bH. M. L. Davies, T. Hansen, J. Rutberg, P. R. Bruzinski, Tetrahedron Lett. 1997, 38, 1741–1744;
- 17cR. T. Buck, D. M. Coe, M. J. Drysdale, L. Ferris, D. Haigh, C. J. Moody, N. D. Pearson, J. B. Sanghera, Tetrahedron: Asymmetry 2003, 14, 791–816;
- 17dD. Chen, D.-X. Zhu, M.-H. Xu, J. Am. Chem. Soc. 2016, 138, 1498–1501; for other relevant examples of Si−H insertions, see:
- 17eY.-Z. Zhang, S.-F. Zhu, L.-X. Wang, Q.-L. Zhou, Angew. Chem. Int. Ed. 2008, 47, 8496–8498; Angew. Chem. 2008, 120, 8624–8626;
- 17fS. B. J. Kan, R. D. Lewis, K. Chen, F. H. Arnold, Science 2016, 354, 1048–1051;
- 17gH. Keipour, T. Ollevier, Org. Lett. 2017, 19, 5736–5739.
- 18For details on reaction development and optimization, see the Supporting Information.
- 19
- 19aS. D. Rychnovsky, Chem. Rev. 1995, 95, 2021–2040;
- 19bC. Thirsk, A. Whiting, J. Chem. Soc. Perkin Trans. 1 2002, 999–1023;
- 19cDictionary of Natural Products Version 27.1 (Taylor and Drancis Group, 2013); http://www.dnp.chemnetbase.com;
- 19dE. M. Woerly, J. Roy, M. D. Burke, Nat. Chem. 2014, 6, 484–491.
- 20
- 20aH. L. Sleeper, W. Fenical, J. Am. Chem. Soc. 1977, 99, 2367–2368;
- 20bM. Sakakibara, M. Matsui, Agric. Biol. Chem. 1979, 43, 117–123;
- 20cD. Soullez, Y. Ramondenc, G. Ple, L. Duhamel, Nat. Prod. Lett. 1994, 4, 203–208.
- 21Y. Landais, L. Parra-Rapado, D. Planchenault, V. Weber, Tetrahedron Lett. 1997, 38, 229–232.