Acetaldehyde Silyl Enol Ethers in Enantioselective Mukaiyama Aldol Reactions: Enzyme-Like Organocatalysis in Action
In memory of Teruaki Mukaiyama (1927–2018)
Graphical Abstract
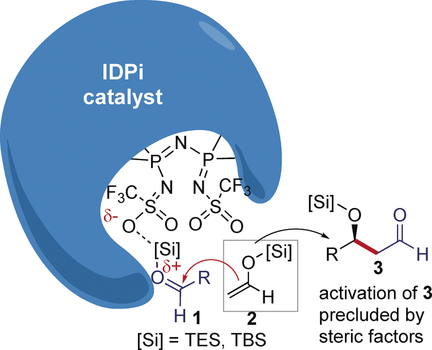
Touched for the very first time! It is herein highlighted how acetaldehyde silyl enol ethers undergo enantioselective Mukaiyama aldol reaction with aliphatic and aromatic aldehydes. The chemistry relies on the use of the highly efficient and substrate-selective imidodiphosphorimidate catalyst, which displays some of the features of enzymatic catalysis.