There and Back Again—The Journey of LiNiO2 as a Cathode Active Material
Corresponding Author
Dr. Matteo Bianchini
Battery and Electrochemistry Laboratory (BELLA), Institute of Nanotechnology, Karlsruhe Institute for Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
Search for more papers by this authorDr. Maria Roca-Ayats
Battery and Electrochemistry Laboratory (BELLA), Institute of Nanotechnology, Karlsruhe Institute for Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
Search for more papers by this authorDr. Pascal Hartmann
Battery and Electrochemistry Laboratory (BELLA), Institute of Nanotechnology, Karlsruhe Institute for Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
BASF SE, 67056 Ludwigshafen, Germany
Search for more papers by this authorDr. Torsten Brezesinski
Battery and Electrochemistry Laboratory (BELLA), Institute of Nanotechnology, Karlsruhe Institute for Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Jürgen Janek
Battery and Electrochemistry Laboratory (BELLA), Institute of Nanotechnology, Karlsruhe Institute for Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
Institute of Physical Chemistry &, Center for Materials Science (ZfM/LaMa), Justus-Liebig-University Giessen, Heinrich-Buff-Ring 17, 35392 Giessen, Germany
Search for more papers by this authorCorresponding Author
Dr. Matteo Bianchini
Battery and Electrochemistry Laboratory (BELLA), Institute of Nanotechnology, Karlsruhe Institute for Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
Search for more papers by this authorDr. Maria Roca-Ayats
Battery and Electrochemistry Laboratory (BELLA), Institute of Nanotechnology, Karlsruhe Institute for Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
Search for more papers by this authorDr. Pascal Hartmann
Battery and Electrochemistry Laboratory (BELLA), Institute of Nanotechnology, Karlsruhe Institute for Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
BASF SE, 67056 Ludwigshafen, Germany
Search for more papers by this authorDr. Torsten Brezesinski
Battery and Electrochemistry Laboratory (BELLA), Institute of Nanotechnology, Karlsruhe Institute for Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Jürgen Janek
Battery and Electrochemistry Laboratory (BELLA), Institute of Nanotechnology, Karlsruhe Institute for Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
Institute of Physical Chemistry &, Center for Materials Science (ZfM/LaMa), Justus-Liebig-University Giessen, Heinrich-Buff-Ring 17, 35392 Giessen, Germany
Search for more papers by this authorGraphical Abstract
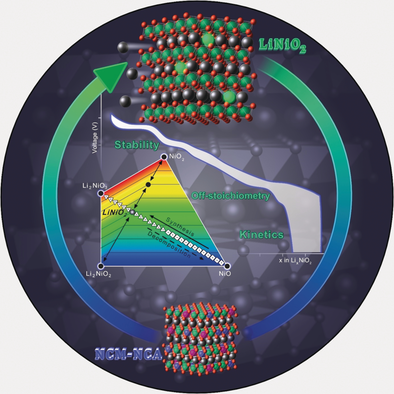
Taking charge: LiNiO2 is one of the most heavily investigated layered oxides for use as the cathode active material of Li-ion batteries. This Review gives a comprehensive overview of this compound, almost 30 years after its introduction. The focus lies in the effect of Li off-stoichiometry, the key instability issues that plague the material and the strategies to overcome them, the open questions, and the most promising future research directions.
Abstract
This Review provides a comprehensive overview of LiNiO2 (LNO), almost 30 years after its introduction as a cathode active material. We aim to highlight the physicochemical peculiarities that make LNO a complex material in every aspect. We specifically stress the effect of the Li off-stoichiometry (Li1−zNi1+zO2) on every property of LNO, especially the electrochemical ones. The key instability issues that plague the compound and the strategies that have been implemented so far to overcome them are discussed in detail. Finally, the open questions that remain to be addressed by the scientific community are summarized, and the research directions that seem the most promising to enable LNO to be fully exploited are elucidated.
Conflict of interest
The authors declare no conflict of interest.
References
- 1G. E. Blomgren, J. Electrochem. Soc. 2017, 164, A 5019–A5025.
- 2M. S. Dresselhaus, G. Dresselhaus, Adv. Phys. 1981, 30, 139–326.
- 3K. Mizushima, P. C. Jones, P. J. Wiseman, J. B. Goodenough, Mater. Res. Bull. 1980, 15, 783–789.
- 4
- 4aZ. Chen, Z. Lu, J. R. Dahn, J. Electrochem. Soc. 2002, 149, A 1604–A1609;
- 4bK. T. Lee, S. Jeong, J. Cho, Acc. Chem. Res. 2013, 46, 1161–1170.
- 5J. R. Dahn, E. W. Fuller, M. Obrovac, U. Vonsacken, Solid State Ionics 1994, 69, 265–270.
- 6E. A. Olivetti, G. Ceder, G. G. Gaustad, X. Fu, Joule 2017, 1, 229–243.
- 7
- 7aJ. R. Dahn, U. Vonsacken, C. A. Michal, Solid State Ionics 1990, 44, 87–97;
- 7bJ. R. Dahn, U. Vonsacken, M. W. Juzkow, H. Aljanaby, J. Electrochem. Soc. 1991, 138, 2207–2211;
- 7cP. G. Bruce, A. Lisowskaoleksiak, M. Y. Saidi, C. A. Vincent, Solid State Ionics 1992, 57, 353–358;
- 7dT. Ohzuku, A. Ueda, M. Nagayama, J. Electrochem. Soc. 1993, 140, 1862–1870;
- 7eT. Ohzuku, A. Ueda, M. Nagayama, Y. Iwakoshi, H. Komori, Electrochim. Acta 1993, 38, 1159–1167.
- 8C. Delmas, J. P. Peres, A. Rougier, A. Demourgues, F. Weill, A. Chadwick, M. Broussely, F. Perton, P. Biensan, P. Willmann, J. Power Sources 1997, 68, 120–125.
- 9C. Delmas, J. J. Braconnier, C. Fouassier, P. Hagenmuller, Solid State Ionics 1981, 3–4, 165–169.
- 10S.-T. Myung, F. Maglia, K.-J. Park, C. S. Yoon, P. Lamp, S.-J. Kim, Y.-K. Sun, ACS Energy Lett. 2017, 2, 196–223.
- 11D. Andre, S.-J. Kim, P. Lamp, S. F. Lux, F. Maglia, O. Paschos, B. Stiaszny, J. Mater. Chem. A 2015, 3, 6709–6732.
- 12
- 12aF. Schipper, E. M. Erickson, C. Erk, J.-Y. Shin, F. F. Chesneau, D. Aurbach, J. Electrochem. Soc. 2017, 164, A 6220–A6228;
- 12bJ. Xu, F. Lin, M. M. Doeff, W. Tong, J. Mater. Chem. A 2017, 5, 874–901;
- 12cW. Liu, P. Oh, X. Liu, M.-J. Lee, W. Cho, S. Chae, Y. Kim, J. Cho, Angew. Chem. Int. Ed. 2015, 54, 4440–4457; Angew. Chem. 2015, 127, 4518–4536;
- 12dL. de Biasi, A. O. Kondrakov, H. Geßwein, T. Brezesinski, P. Hartmann, J. Janek, J. Phys. Chem. C 2017, 121, 26163–26171;
- 12eY.-K. Sun, S.-T. Myung, B.-C. Park, J. Prakash, I. Belharouak, K. Amine, Nat. Mater. 2009, 8, 320–324;
- 12fW. Li, B. Song, A. Manthiram, Chem. Soc. Rev. 2017, 46, 3006–3059.
- 13L. D. Dyer, B. S. Borie, G. P. Smith, J. Am. Chem. Soc. 1954, 76, 1499–1503.
- 14
- 14aJ. B. Goodenough, D. G. Wickham, W. J. Croft, J. Phys. Chem. Solids 1958, 5, 107–116;
- 14bJ. B. Goodenough, D. G. Wickham, W. J. Croft, J. Appl. Phys. 1958, 29, 382–383.
- 15A. Rougier, I. Saadoune, P. Gravereau, P. Willmann, C. Delmas, Solid State Ionics 1996, 90, 83–90.
- 16R. Shannon, Acta Crystallogr. Sect. A 1976, 32, 751–767.
- 17A. Rougier, P. Gravereau, C. Delmas, J. Electrochem. Soc. 1996, 143, 1168–1175.
- 18R. Kanno, H. Kubo, Y. Kawamoto, T. Kamiyama, F. Izumi, Y. Takeda, M. Takano, J. Solid State Chem. 1994, 110, 216–225.
- 19A. Van der Ven, G. Ceder, Electrochem. Solid-State Lett. 2000, 3, 301–304.
- 20
- 20aC. Delmas, M. Menetrier, L. Croguennec, I. Saadoune, A. Rougier, C. Pouillerie, G. Prado, M. Grune, L. Fournes, Electrochim. Acta 1999, 45, 243–253;
- 20bP. Kalyani, N. Kalaiselvi, Sci. Technol. Adv. Mater. 2005, 6, 689–703.
- 21
- 21aC. Pouillerie, E. Suard, C. Delmas, J. Solid State Chem. 2001, 158, 187–197;
- 21bA. Hirano, R. Kanno, Y. Kawamoto, Y. Takeda, K. Yamaura, M. Takano, K. Ohyama, M. Ohashi, Y. Yamaguchi, Solid State Ionics 1995, 78, 123–131.
- 22
- 22aI. J. Pickering, J. T. Lewandowski, A. J. Jacobson, J. A. Goldstone, Solid State Ionics 1992, 53, 405–412;
- 22bW. Bronger, H. Bade, W. Klemm, Z. Anorg. Allg. Chem. 1964, 333, 188–200.
- 23J. H. Chung, T. Proffen, S. Shamoto, A. M. Ghorayeb, L. Croguennec, W. Tian, B. C. Sales, R. Jin, D. Mandrus, T. Egami, Phys. Rev. B 2005, 71, 064410.
- 24
- 24aY. Kim, J. Mol. Struct. 2015, 1099, 317–322;
- 24bH. Das, A. Urban, W. Huang, G. Ceder, Chem. Mater. 2017, 29, 7840–7851.
- 25
- 25aR. J. Moore, J. White, J. Mater. Sci. 1974, 9, 1401–1408;
- 25bH. N. Migeon, M. Zanne, C. Gleitzer, J. Aubry, J. Mater. Sci. 1978, 13, 461–466.
- 26J. M. Tarascon, G. Vaughan, Y. Chabre, L. Seguin, M. Anne, P. Strobel, G. Amatucci, J. Solid State Chem. 1999, 147, 410–420.
- 27H. Migeon, A. Courtois, M. Zanne, C. Gleitzer, Rev. Chim. Miner. 1976, 13, 1–8.
- 28
- 28aR. Stoyanova, E. Zhecheva, R. Alcántara, J. L. Tirado, G. Bromiley, F. Bromiley, T. Boffa Ballaran, Solid State Ionics 2003, 161, 197–204;
- 28bE. Shinova, E. Zhecheva, R. Stoyanova, G. D. Bromiley, J. Solid State Chem. 2005, 178, 1661–1669.
- 29
- 29aM. Tabuchi, N. Kuriyama, K. Takamori, Y. Imanari, K. Nakane, J. Electrochem. Soc. 2016, 163, A 2312–A2317;
- 29bY. Kobayashi, M. Tabuchi, H. Miyashiro, N. Kuriyama, J. Power Sources 2017, 364, 156–162.
- 30
- 30aV. Berbenni, V. Massarotti, D. Capsoni, R. Riccardi, A. Marini, E. Antolini, Solid State Ionics 1991, 48, 101–111;
- 30bW. Li, J. N. Reimers, J. R. Dahn, Phys. Rev. B 1992, 46, 3236–3246.
- 31E. Antolini, Mater. Chem. Phys. 2003, 82, 937–948.
- 32E. McCalla, G. H. Carey, J. R. Dahn, Solid State Ionics 2012, 219, 11–19.
- 33
- 33aH. Arai, M. Tsuda, Y. Sakurai, J. Power Sources 2000, 90, 76–81;
- 33bL. Croguennec, E. Suard, P. Willmann, C. Delmas, Chem. Mater. 2002, 14, 2149–2157;
- 33cK. Mukai, J. Sugiyama, Y. Aoki, J. Solid State Chem. 2010, 183, 1726–1732.
- 34H. Arai, S. Okada, H. Ohtsuka, M. Ichimura, J. Yamaki, Solid State Ionics 1995, 80, 261–269.
- 35K. Hoang, M. D. Johannes, J. Mater. Chem. A 2014, 2, 5224–5235.
- 36Y. Koyama, H. Arai, I. Tanaka, Y. Uchimoto, Z. Ogumi, Chem. Mater. 2012, 24, 3886–3894.
- 37R. V. Moshtev, P. Zlatilova, V. Manev, A. Sato, J. Power Sources 1995, 54, 329–333.
- 38J. N. Reimers, J. R. Dahn, J. E. Greedan, C. V. Stager, G. Liu, I. Davidson, U. Vonsacken, J. Solid State Chem. 1993, 102, 542–552.
- 39
- 39aS. P. Lin, K. Z. Fung, Y. M. Hon, M. H. Hon, J. Cryst. Growth 2002, 234, 176–183;
- 39bM. Balandeh, S. Asgari, J. Nanomater. 2010, 2010, 1–6;
- 39cM. Song, S. Kwon, I. Kwon, H. Park, J. Appl. Electrochem. 2007, 37, 421–427;
- 39dP. Mohan, G. P. Kalaignan, J. Electroceram. 2013, 31, 210–217;
- 39eS. N. Kwon, D. R. Mumm, H. R. Park, M. Y. Song, J. Ceram. Process. Res. 2016, 17, 653–658.
- 40
- 40aC.-C. Chang, J. Y. Kim, P. N. Kumta, J. Electrochem. Soc. 2002, 149, A 1114–A1120;
- 40bB. Garcia, J. P. Pereira-Ramos, D. Caurant, N. Baffier, Chem. Lett. 1998, 27, 543–544;
- 40cL. Croguennec, C. Pouillerie, A. N. Mansour, C. Delmas, J. Mater. Chem. 2001, 11, 131–141.
- 41A. Rougier, Université Sciences et Technologies—Bordeaux I 1995.
- 42M. G. S. R. Thomas, W. I. F. David, J. B. Goodenough, P. Groves, Mater. Res. Bull. 1985, 20, 1137–1146.
- 43H. Rieck, R. Hoppe, Z. Anorg. Allg. Chem. 1972, 392, 193–196.
- 44I. Davidson, J. E. Greedan, U. Vonsacken, C. A. Michal, J. R. Dahn, Solid State Ionics 1991, 46, 243–247.
- 45T. A. Hewston, B. L. Chamberland, J. Phys. Chem. Solids 1987, 48, 97–108.
- 46
- 46aA. Rougier, C. Delmas, A. V. Chadwick, Solid State Commun. 1995, 94, 123–127;
- 46bD. G. Kellerman, Russ. Chem. Rev. 2001, 70, 777–790.
- 47I. Nakai, K. Takahashi, Y. Shiraishi, T. Nakagome, F. Nishikawa, J. Solid State Chem. 1998, 140, 145–148.
- 48
- 48aZ. Chen, H. Zou, X. Zhu, J. Zou, J. Cao, J. Solid State Chem. 2011, 184, 1784–1790;
- 48bH. Chen, C. L. Freeman, J. H. Harding, Phys. Rev. B 2011, 84, 085108.
- 49J. Cao, H. Zou, C. Guo, Z. Chen, S. Pu, Solid State Ionics 2009, 180, 1209–1214.
- 50L. A. Montoro, M. Abbate, E. C. Almeida, J. M. Rosolen, Chem. Phys. Lett. 1999, 309, 14–18.
- 51M. K. Aydinol, A. F. Kohan, G. Ceder, K. Cho, J. Joannopoulos, Phys. Rev. B 1997, 56, 1354–1365.
- 52
- 52aJ. P. Kemp, P. A. Cox, J. W. Hodby, J. Phys. Condens. Matter 1990, 2, 6699;
- 52bK. Hirakawa, H. Kadowaki, K. Ubukoshi, J. Phys. Soc. Jpn. 1985, 54, 3526–3536;
- 52cK. Hirakawa, H. Kadowaki, Phys. B+C 1986, 136, 335–340;
- 52dT. Shirakami, M. Takematsu, A. Hirano, R. Kanno, K. Yamaura, M. Takano, T. Atake, Mater. Sci. Eng. B 1998, 54, 70–72;
- 52eK. Hirota, Y. Nakazawa, M. Ishikawa, J. Phys. Condens. Matter 1991, 3, 4721.
- 53
- 53aA. Rougier, C. Delmas, G. Chouteau, J. Phys. Chem. Solids 1996, 57, 1101–1103;
- 53bK. Yamaura, M. Takano, A. Hirano, R. Kanno, J. Solid State Chem. 1996, 127, 109–118.
- 54
- 54aD. Mertz, Y. Ksari, F. Celestini, J. M. Debierre, A. Stepanov, C. Delmas, Phys. Rev. B 2000, 61, 1240–1245;
- 54bA. L. Barra, G. Chouteau, A. Stepanov, C. Delmas, J. Magn. Magn. Mater. 1998, 177, 783–784.
- 55C. Pouillerie, L. Croguennec, P. Biensan, P. Willmann, C. Delmas, J. Electrochem. Soc. 2000, 147, 2061–2069.
- 56M. Wang, A. Navrotsky, Solid State Ionics 2004, 166, 167–173.
- 57H. Yokokawa, N. Sakai, K. Yamaji, T. Horita, M. Ishikawa, Solid State Ionics 1998, 113–115, 1–9.
- 58K. Chang, B. Hallstedt, D. Music, CALPHAD Comput. Coupling Phase Diagrams Thermochem 2012, 37, 100–107.
- 59H. Kawaji, T. Oka, T. Tojo, T. Atake, A. Hirano, R. Kanno, Solid State Ionics 2002, 152–153, 195–198.
- 60J. Akimoto, Y. Gotoh, Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A 2000, 341, 143–146.
- 61
- 61aK. S. Park, S. H. Park, Y. K. Sun, K. S. Nahm, Y. S. Lee, M. Yoshio, J. Appl. Electrochem. 2002, 32, 1229–1233;
- 61bC.-H. Lu, L. Wei-Cheng, J. Mater. Chem. 2000, 10, 1403–1407.
- 62S. Yamada, M. Fujiwara, M. Kanda, J. Power Sources 1995, 54, 209–213.
- 63T. Sata, Ceram. Int. 1998, 24, 53–59.
- 64J. Xu, F. Lin, D. Nordlund, E. J. Crumlin, F. Wang, J. Bai, M. M. Doeff, W. Tong, Chem. Commun. 2016, 52, 4239–4242.
- 65
- 65aC. K. Back, R.-Z. Yin, S.-J. Shin, Y.-S. Lee, W. Choi, Y.-S. Kim, J. Electrochem. Soc. 2012, 159, A 887–A893;
- 65bK. Kang, C.-H. Chen, B. J. Hwang, G. Ceder, Chem. Mater. 2004, 16, 2685–2690.
- 66
- 66aD. Caurant, N. Baffier, B. Garcia, J. P. PereiraRamos, Solid State Ionics 1996, 91, 45–54;
- 66bM. Guilmard, A. Rougier, M. Grüne, L. Croguennec, C. Delmas, J. Power Sources 2003, 115, 305–314;
- 66cU. H. Kim, D. W. Jun, K. J. Park, Q. Zhang, P. Kaghazchi, D. Aurbach, D. T. Major, G. Goobes, M. Dixit, N. Leifer, C. M. Wang, P. Yan, D. Ahn, K. H. Kim, C. S. Yoon, Y. K. Sun, Energy Environ. Sci. 2018, 11, 1271–1279.
- 67
- 67aS. Albrecht, M. Kruft, S. Malcus, Google Patents, US20090302267A1, 2009;
- 67bV. Stoller, A. Olbrich, J. Meese-Marktscheffel, M. Wohlfahrt-Mehrens, P. Axmann, H. Dittrich, S. Ströbele, CA2356984C, 1999.
- 68M. H. Lee, Y. Kang, S. T. Myung, Y. K. Sun, Electrochim. Acta 2004, 50, 939–948.
- 69C.-C. Chang, N. Scarr, P. N. Kumta, Solid State Ionics 1998, 112, 329–344.
- 70Y. S. Lee, Y. K. Sun, K. S. Nahm, Solid State Ionics 1999, 118, 159–168.
- 71M. Y. Song, R. Lee, J. Power Sources 2002, 111, 97–103.
- 72Y.-K. Sun, I.-H. Oh, J. Mater. Sci. Lett. 1997, 16, 30–32.
- 73
- 73aC.-C. Chang, J. Y. Kim, P. N. Kumta, J. Electrochem. Soc. 2002, 149, A 331–A338;
- 73bC.-C. Chang, P. N. Kumta, J. Power Sources 1998, 75, 44–55.
- 74C. Dearden, M. Zhu, B. Wang, R. H. R. Castro, J. Mater. Sci. 2013, 48, 1740–1745.
- 75
- 75aN. Kalaiselvi, A. V. Raajaraajan, B. Sivagaminathan, N. G. Renganathan, N. Muniyandi, M. Ragavan, Ionics 2003, 9, 382–387;
- 75bM. Y. Song, I. H. Kwon, H. R. Park, D. R. Mumm, Ceram. Int. 2012, 38, 2443–2448.
- 76
- 76aM. M. Rao, C. Liebenow, M. Jayalakshmi, H. Wulff, U. Guth, F. Scholz, J. Solid State Electrochem. 2001, 5, 348–354;
- 76bM. Song, I. Kwon, H. Kim, S. Shim, D. R. Mumm, J. Appl. Electrochem. 2006, 36, 801–805.
- 77
- 77aD. Larcher, M. R. Palacin, G. G. Amatucci, J. M. Tarascon, J. Electrochem. Soc. 1997, 144, 408–417;
- 77bX. Lai, D. Gao, J. Bi, Y. Li, P. Cheng, C. Xu, D. Lin, J. Alloys Compd. 2009, 487, L30–L32;
- 77cM. R. Palacín, D. Larcher, A. Audemer, N. Sac-Épee, G. G. Amatucci, J. M. Tarascon, J. Electrochem. Soc. 1997, 144, 4226–4236;
- 77dY. Sun, P. Wan, J. Pan, C. Xu, X. Liu, Solid State Ionics 2006, 177, 1173–1177.
- 78
- 78aP. Kalyani, N. Kalaiselvi, N. G. Renganathan, J. Power Sources 2003, 123, 53–60;
- 78bA. Vandenberg, A. Hintennach, Russian J. Electrochem. 2015, 51, 310–317.
- 79C.-C. Chang, P. N. Kumta, Mater. Sci. Eng. B 2005, 116, 341–345.
- 80
- 80aM. A. Eleruja, G. O. Egharevba, O. A. Abulude, O. O. Akinwunmi, C. Jeynes, E. O. B. Ajayi, J. Mater. Sci. 2007, 42, 2758–2765;
- 80bM. Rubin, S. J. Wen, T. Richardson, J. Kerr, K. von Rottkay, J. Slack, Solar Energy Mater. Solar Cells 1998, 54, 59–66.
- 81
- 81aA. Mansour, Surf. Sci. Spectra 1994, 3, 279–286;
- 81bM. Oku, H. Tokuda, K. Hirokawa, J. Electron Spectrosc. Relat. Phenom. 1991, 53, 201–211.
- 82
- 82aG. V. Zhuang, G. Chen, J. Shim, X. Song, P. N. Ross, T. J. Richardson, J. Power Sources 2004, 134, 293–297;
- 82bK. Matsumoto, R. Kuzuo, K. Takeya, A. Yamanaka, J. Power Sources 1999, 81, 558–561;
- 82cT. Hatsukade, A. Schiele, P. Hartmann, T. Brezesinski, J. Janek, ACS Appl. Mater. Interfaces 2018, 10, 38892–38899.
- 83
- 83aJ. R. Dahn, R. Fong, U. Von Sacken, US5264201, 1993;
- 83bJ. Kim, Y. S. Hong, K. S. Ryu, M. G. Kim, J. Cho, Electrochem. Solid-State Lett. 2006, 9, A 19–A23.
- 84R. Moshtev, P. Zlatilova, S. Vasilev, I. Bakalova, A. Kozawa, J. Power Sources 1999, 81, 434–441.
- 85I. A. Shkrob, J. A. Gilbert, P. J. Phillips, R. Klie, R. T. Haasch, J. Bareño, D. P. Abraham, J. Electrochem. Soc. 2017, 164, A 1489–A1498.
- 86
- 86aC. S. Yoon, D.-W. Jun, S.-T. Myung, Y.-K. Sun, ACS Energy Lett. 2017, 2, 1150–1155;
- 86bJ. Xu, E. Hu, D. Nordlund, A. Mehta, S. N. Ehrlich, X.-Q. Yang, W. Tong, ACS Appl. Mater. Interfaces 2016, 8, 31677–31683;
- 86cH. Li, N. Zhang, J. Li, J. R. Dahn, J. Electrochem. Soc. 2018, 165, A 2985–A2993.
- 87M. Broussely, F. Perton, P. Biensan, J. M. Bodet, J. Labat, A. Lecerf, C. Delmas, A. Rougier, J. P. Peres, J. Power Sources 1995, 54, 109–114.
- 88D. D. MacNeil, Z. Lu, Z. Chen, J. R. Dahn, J. Power Sources 2002, 108, 8–14.
- 89C. Delmas, M. Menetrier, L. Croguennec, S. Levasseur, J. P. Peres, C. Pouillerie, G. Prado, L. Fournes, F. Weill, Int. J. Inorg. Mater. 1999, 1, 11–19.
- 90W. Li, J. N. Reimers, J. R. Dahn, Solid State Ionics 1993, 67, 123–130.
- 91H. Arai, S. Okada, Y. Sakurai, J. Yamaki, Solid State Ionics 1997, 95, 275–282.
- 92J. P. Peres, C. Delmas, A. Rougier, M. Broussely, F. Perton, P. Biensan, P. Willmann, J. Phys. Chem. Solids 1996, 57, 1057–1060.
- 93K. Kang, G. Ceder, Phys. Rev. B 2006, 74, 094105.
- 94A. Van der Ven, J. C. Thomas, Q. Xu, B. Swoboda, D. Morgan, Phys. Rev. B 2008, 78, 104306.
- 95M. E. Arroyo y de Dompablo, A. Van der Ven, G. Ceder, Phys. Rev. B 2002, 66, 064112.
- 96
- 96aE. Levi, M. D. Levi, G. Salitra, D. Aurbach, R. Oesten, U. Heider, L. Heider, Solid State Ionics 1999, 126, 97–108;
- 96bX. Q. Yang, X. Sun, J. McBreen, Electrochem. Commun. 1999, 1, 227–232.
- 97K. Y. Chung, W.-S. Yoon, H. S. Lee, J. McBreen, X.-Q. Yang, S. H. Oh, W. H. Ryu, J. L. Lee, W. I. Cho, B. W. Cho, J. Power Sources 2006, 163, 185–190.
- 98K. Dokko, M. Nishizawa, S. Horikoshi, T. Itoh, M. Mohamedi, I. Uchida, Electrochem. Solid-State Lett. 2000, 3, 125–127.
- 99
- 99aJ. P. Peres, A. Demourgues, C. Delmas, Solid State Ionics 1998, 111, 135–144;
- 99bA. N. Mansour, X. Q. Yang, X. Sun, J. McBreen, L. Croguennec, C. Delmas, J. Electrochem. Soc. 2000, 147, 2104–2109.
- 100J. P. Peres, F. Weill, C. Delmas, Solid State Ionics 1999, 116, 19–27.
- 101J.-P. Peres, PhD Thesis, Le système LixNiO2: de l′électrochimie à la cristallographie, 1996.
- 102
- 102aM. E. Arroyo y de Dompablo, G. Ceder, J. Power Sources 2003, 119–121, 654–657;
- 102bM. E. Arroyo y de Dompablo, G. Ceder, Chem. Mater. 2003, 15, 63–67.
- 103
- 103aL. Croguennec, C. Pouillerie, C. Delmas, J. Electrochem. Soc. 2000, 147, 1314–1321;
- 103bL. Croguennec, C. Pouillerie, C. Delmas, Solid State Ionics 2000, 135, 259–266;
- 103cL. Seguin, G. Amatucci, M. Anne, Y. Chabre, P. Strobel, J. M. Tarascon, G. Vaughan, J. Power Sources 1999, 81, 604–606.
- 104G. G. Amatucci, J. M. Tarascon, L. C. Klein, J. Electrochem. Soc. 1996, 143, 1114–1123.
- 105H. Chen, J. A. Dawson, J. H. Harding, J. Mater. Chem. A 2014, 2, 7988–7996.
- 106
- 106aH.-H. Sun, A. Manthiram, Chem. Mater. 2017, 29, 8486–8493;
- 106bA. O. Kondrakov, A. Schmidt, J. Xu, H. Geßwein, R. Mönig, P. Hartmann, H. Sommer, T. Brezesinski, J. Janek, J. Phys. Chem. C 2017, 121, 3286–3294.
- 107
- 107aP. Yan, J. Zheng, M. Gu, J. Xiao, J.-G. Zhang, C.-M. Wang, Nat. Commun. 2017, 8, 14101;
- 107bJ.-M. Lim, T. Hwang, D. Kim, M.-S. Park, K. Cho, M. Cho, Sci. Rep. 2017, 7, 39669;
- 107cA. O. Kondrakov, H. Geßwein, K. Galdina, L. de Biasi, V. Meded, E. O. Filatova, G. Schumacher, W. Wenzel, P. Hartmann, T. Brezesinski, J. Janek, J. Phys. Chem. C 2017, 121, 24381–24388.
- 108J. Morales, C. Pérez-Vicente, J. L. Tirado, J. Therm. Anal. 1992, 38, 295–301.
- 109H. Arai, S. Okada, Y. Sakurai, J.-i. Yamaki, Solid State Ionics 1998, 109, 295–302.
- 110
- 110aT. Ohzuku, A. Ueda, M. Kouguchi, J. Electrochem. Soc. 1995, 142, 4033–4039;
- 110bM. Guilmard, L. Croguennec, D. Denux, C. Delmas, Chem. Mater. 2003, 15, 4476–4483.
- 111D. Wainwright, J. Power Sources 1995, 54, 192–197.
- 112Z. Zhang, D. Fouchard, J. R. Rea, J. Power Sources 1998, 70, 16–20.
- 113W. Li, J. C. Currie, J. Wolstenholme, J. Power Sources 1997, 68, 565–569.
- 114
- 114aC. H. Chen, J. Liu, K. Amine, J. Power Sources 2001, 96, 321–328;
- 114bD. P. Abraham, R. D. Twesten, M. Balasubramanian, I. Petrov, J. McBreen, K. Amine, Electrochem. Commun. 2002, 4, 620–625;
- 114cA. M. Andersson, D. P. Abraham, R. Haasch, S. MacLaren, J. Liu, K. Amine, J. Electrochem. Soc. 2002, 149, A 1358–A1369;
- 114dM. Balasubramanian, H. S. Lee, X. Sun, X. Q. Yang, A. R. Moodenbaugh, J. McBreen, D. A. Fischer, Z. Fu, Electrochem. Solid-State Lett. 2002, 5, A 22–A25;
- 114eD. P. Abraham, J. Liu, C. H. Chen, Y. E. Hyung, M. Stoll, N. Elsen, S. MacLaren, R. Twesten, R. Haasch, E. Sammann, I. Petrov, K. Amine, G. Henriksen, J. Power Sources 2003, 119–121, 511–516;
- 114fD. P. Abraham, R. D. Twesten, M. Balasubramanian, J. Kropf, D. Fischer, J. McBreen, I. Petrov, K. Amine, J. Electrochem. Soc. 2003, 150, A 1450–A1456.
- 115
- 115aF. Schipper, E. M. Erickson, C. Erk, J.-Y. Shin, F. F. Chesneau, D. Aurbach, J. Electrochem. Soc. 2017, 164, A 6220–A6228;
- 115bT. Sasaki, T. Nonaka, H. Oka, C. Okuda, Y. Itou, Y. Kondo, Y. Takeuchi, Y. Ukyo, K. Tatsumi, S. Muto, J. Electrochem. Soc. 2009, 156, A 289–A293;
- 115cS. Muto, Y. Sasano, K. Tatsumi, T. Sasaki, K. Horibuchi, Y. Takeuchi, Y. Ukyo, J. Electrochem. Soc. 2009, 156, A 371–A377.
- 116F. Lin, I. M. Markus, D. Nordlund, T.-C. Weng, M. D. Asta, H. L. Xin, M. M. Doeff, Nat. Commun. 2014, 5, 3529.
- 117H. Zhang, B. M. May, J. Serrano-Sevillano, M. Casas-Cabanas, J. Cabana, C. Wang, G. Zhou, Chem. Mater. 2018, 30, 692–699.
- 118A. Urban, J. Lee, G. Ceder, Adv. Energy Mater. 2014, 4, 1400478.
- 119E. Cho, S. W. Seo, K. Min, ACS Appl. Mater. Interfaces 2017, 9, 33257–33266.
- 120R. Jung, M. Metzger, F. Maglia, C. Stinner, H. A. Gasteiger, J. Electrochem. Soc. 2017, 164, A 1361–A1377.
- 121D. Aurbach, J. Power Sources 2003, 119–121, 497–503.
- 122H. S. Liu, Z. R. Zhang, Z. L. Gong, Y. Yang, Electrochem. Solid-State Lett. 2004, 7, A 190–A193.
- 123E. Rossen, C. D. W. Jones, J. R. Dahn, Solid State Ionics 1992, 57, 311–318.
- 124
- 124aE. Zhecheva, R. Stoyanova, Solid State Ionics 1993, 66, 143–149;
- 124bR. J. Gummow, M. M. Thackeray, J. Electrochem. Soc. 1993, 140, 3365–3368.
- 125J. N. Reimers, E. Rossen, C. D. Jones, J. R. Dahn, Solid State Ionics 1993, 61, 335–344.
- 126
- 126aY. P. Wu, E. Rahm, R. Holze, Electrochim. Acta 2002, 47, 3491–3507;
- 126bC. Delmas, L. Croguennec, MRS Bull. 2002, 27, 608–612.
- 127C. D. W. Jones, E. Rossen, J. R. Dahn, Solid State Ionics 1994, 68, 65–69.
- 128
- 128aC. Marichal, J. Hirschinger, P. Granger, M. Menetrier, A. Rougier, C. Delmas, Inorg. Chem. 1995, 34, 1773–1778;
- 128bW. Li, J. C. Currie, J. Electrochem. Soc. 1997, 144, 2773–2779;
- 128cI. Saadoune, C. Delmas, J. Mater. Chem. 1996, 6, 193–199;
- 128dJ. Cho, B. Park, J. Power Sources 2001, 92, 35–39;
- 128eG. M. Ehrlich, F. J. Puglia, R. Gitzendanner, B. Hellen, C. Marsh, J. Power Sources 1999, 81–82, 863–866.
- 129
- 129aB. Fuchs, S. Kemmler-Sack, Solid State Ionics 1994, 68, 279–285;
- 129bR. Kanno, T. Shirane, Y. Inaba, Y. Kawamoto, J. Power Sources 1997, 68, 145–152;
- 129cG. Prado, E. Suard, L. Fournes, C. Delmas, J. Mater. Chem. 2000, 10, 2553–2560;
- 129dG. Prado, A. Rougier, L. Fournès, C. Delmas, J. Electrochem. Soc. 2000, 147, 2880–2887.
- 130
- 130aE. McCalla, J. R. Dahn, Solid State Ionics 2013, 242, 1–9;
- 130bA. R. Armstrong, P. G. Bruce, Nature 1996, 381, 499;
- 130cR. Hoppe, G. Brachtel, M. Jansen, Z. Anorg. Allg. Chem. 1975, 417, 1–10;
- 130dY. Nitta, K. Okamura, K. Haraguchi, S. Kobayashi, A. Ohata, J. Power Sources 1995, 54, 511–515;
- 130eH. Arai, S. Okada, Y. Sakurai, J. i. Yamaki, J. Electrochem. Soc. 1997, 144, 3117–3125;
- 130fM. Guilmard, L. Croguennec, C. Delmas, Chem. Mater. 2003, 15, 4484–4493.
- 131
- 131aS. Ho Chang, S.-G. Kang, S.-W. Song, J.-B. Yoon, J.-H. Choy, Solid State Ionics 1996, 86–88, 171–175;
- 131bJ. Kim, K. Amine, Electrochem. Commun. 2001, 3, 52–55;
- 131cS. N. Kwon, H. R. Park, M. Y. Song, Ceram. Int. 2014, 40, 11131–11137;
- 131dH.-W. Ha, K. H. Jeong, K. Kim, J. Power Sources 2006, 161, 606–611.
- 132
- 132aL. Croguennec, Y. Shao-Horn, A. Gloter, C. Colliex, M. Guilmard, F. Fauth, C. Delmas, Chem. Mater. 2009, 21, 1051–1059;
- 132bS.-P. Lin, K.-Z. Fung, Y.-M. Hon, M.-H. Hon, J. Solid State Chem. 2002, 167, 97–106;
- 132cZ. Liu, H. Zhen, Y. Kim, C. Liang, J. Power Sources 2011, 196, 10201–10206;
- 132dS. N. Kwon, M. Y. Song, H. R. Park, Ceram. Int. 2014, 40, 14141–14147;
- 132eC. Julien, G. A. Nazri, A. Rougier, Solid State Ionics 2000, 135, 121–130;
- 132fH. R. Park, J. Ind. Eng. Chem. 2010, 16, 698–702;
- 132gY. I. Jang, B. Y. Huang, H. F. Wang, G. R. Maskaly, G. Ceder, D. R. Sadoway, Y. M. Chiang, H. Liu, H. Tamura, J. Power Sources 1999, 81, 589–593;
- 132hG. Ceder, Y. M. Chiang, D. R. Sadoway, M. K. Aydinol, Y. I. Jang, B. Huang, Nature 1998, 392, 694–696.
- 133
- 133aM. Bonda, M. Holzapfel, S. de Brion, C. Darie, T. Fehér, P. J. Baker, T. Lancaster, S. J. Blundell, F. L. Pratt, Phys. Rev. B 2008, 78, 104409;
- 133bC. Pouillerie, L. Croguennec, C. Delmas, Solid State Ionics 2000, 132, 15–29;
- 133cR. Sathiyamoorthi, P. Shakkthivel, S. Ramalakshmi, Y.-G. Shul, J. Power Sources 2007, 171, 922–927;
- 133dP. T. Onnerud, J. J. Shi, S. L. Dalton-Castor, C. Lampe-Onnerud, CA2565810C, 2005;
- 133eY. A. Gao, M. V. Yakovleva, W. B. Ebner, Electrochem. Solid-State Lett. 1999, 1, 117–119.
10.1149/1.1390656 Google Scholar
- 134
- 134aY. Nishida, Y. Nakane, T. Satoh, J. Power Sources 1997, 68, 561–564;
- 134bM. Song, C. Park, S. Yoon, H. Park, D. R. Mumm, Ceram. Int. 2009, 35, 1145–1150;
- 134cM. Song, S. Kwon, H. Park, Ceram. Int. 2009, 35, 3135–3141.
- 135
- 135aJ. Kim, K. Amine, J. Power Sources 2002, 104, 33–39;
- 135bP. Mohan, G. P. Kalaignan, V. S. Muralidharan, J. Nanosci. Nanotechnol. 2012, 12, 7052–7059;
- 135cT. Kudo, N. Mizutani, Key Eng. Mater. 1999, 169–170, 217–220;
10.4028/www.scientific.net/KEM.169-170.217 Google Scholar
- 135dZ. Yang, Z. Zhang, Y. Pan, S. Zhao, Y. Huang, X. Wang, X. Chen, S. Wei, J. Solid State Chem. 2016, 244, 52–60;
- 135eP. Cui, Z. Jia, L. Li, T. He, J. Phys. Chem. Solids 2011, 72, 899–903;
- 135fI. H. Kwon, H. R. Park, Y. Y. Song, Russ. J. Electrochem. 2013, 49, 221–227;
- 135gY. Sato, T. Koyano, M. Mukai, K. Kobayakawa, in Proceedings of the Symposium on Batteries for Portable Applications and Electric Vehicles, Vol. 18, The Electrochemical Society, 1997, p. 45;
- 135hC. S. Yoon, M.-J. Choi, D.-W. Jun, Q. Zhang, P. Kaghazchi, K.-H. Kim, Y.-K. Sun, Chem. Mater. 2018, 30, 1808–1814;
- 135iC. S. Yoon, U.-H. Kim, G.-T. Park, S. J. Kim, K.-H. Kim, J. Kim, Y.-K. Sun, ACS Energy Lett. 2018, 3, 1634–1639.
- 136
- 136aI. Saadoune, M. Ménétrier, C. Delmas, J. Mater. Chem. 1997, 7, 2505–2511;
- 136bI. Saadoune, C. Delmas, J. Solid State Chem. 1998, 136, 8–15.
- 137M. Jansen, R. Hoppe, Z. Anorg. Allg. Chem. 1973, 397, 279–289.
- 138
- 138aK. Kubo, M. Fujiwara, S. Yamada, S. Arai, M. Kanda, J. Power Sources 1997, 68, 553–557;
- 138bK. Kubo, S. Arai, S. Yamada, M. Kanda, J. Power Sources 1999, 81, 599–603.
- 139A. R. Naghash, J. Y. Lee, Electrochim. Acta 2001, 46, 941–951.
- 140A. R. Naghash, J. Y. Lee, Electrochim. Acta 2001, 46, 2293–2304.
- 141
- 141aS. H. Park, Y.-K. Sun, K. S. Park, K. S. Nahm, Y. S. Lee, M. Yoshio, Electrochim. Acta 2002, 47, 1721–1726;
- 141bS. H. Park, K. S. Park, M. H. Cho, Y. K. Sun, K. S. Nahm, Y. S. Lee, M. Yoshio, Korean J. Chem. Eng. 2002, 19, 791–796.
- 142F. Kong, C. Liang, R. C. Longo, D.-H. Yeon, Y. Zheng, J.-H. Park, S.-G. Doo, K. Cho, Chem. Mater. 2016, 28, 6942–6952.
- 143
- 143aC. Li, H. P. Zhang, L. J. Fu, H. Liu, Y. P. Wu, E. Rahm, R. Holze, H. Q. Wu, Electrochim. Acta 2006, 51, 3872–3883;
- 143bS. Neudeck, F. Walther, T. Bergfeldt, C. Suchomski, M. Rohnke, P. Hartmann, J. Janek, T. Brezesinski, ACS Appl. Mater. Interfaces 2018, 10, 20487–20498.
- 144J. Cho, T.-J. Kim, Y. J. Kim, B. Park, Electrochem. Solid-State Lett. 2001, 4, A 159–A161.
- 145
- 145aP. Mohan, G. P. Kalaignan, J. Nanosci. Nanotechnol. 2013, 13, 2765–2770;
- 145bP. Mohan, G. P. Kalaignan, Ionics 2013, 19, 895–902.
- 146J. Kang, B. Han, ACS Appl. Mater. Interfaces 2015, 7, 11599–11603.
- 147D.-W. Jun, C. S. Yoon, U.-H. Kim, Y.-K. Sun, Chem. Mater. 2017, 29, 5048–5052.
- 148
- 148aY. K. Sun, Z. H. Chen, H. J. Noh, D. J. Lee, H. G. Jung, Y. Ren, S. Wang, C. S. Yoon, S. T. Myung, K. Amine, Nat. Mater. 2012, 11, 942–947;
- 148bS. T. Myung, H. J. Noh, S. J. Yoon, E. J. Lee, Y. K. Sun, J. Phys. Chem. Lett. 2014, 5, 671–679.
- 149
- 149aS.-w. Zhong, Y.-j. Zhao, F. Lian, Y. Li, Y. Hu, P. − z. Li, J. Mei, Q.-g. Liu, Trans. Nonferrous Met. Soc. China 2006, 16, 137–141;
- 149bX.-r. Deng, G.-r. Hu, K. Du, Z.-d. Peng, X.-G. Gao, Y.-N. Yang, Mater. Chem. Phys. 2008, 109, 469–474.
- 150
- 150aJ. Lee, A. Urban, X. Li, D. Su, G. Hautier, G. Ceder, Science 2014, 343, 519–522;
- 150bJ. Lee, D. A. Kitchaev, D. H. Kwon, C. W. Lee, J. K. Papp, Y. S. Liu, Z. Y. Lun, R. J. Clement, T. Shi, B. D. McCloskey, J. H. Guo, M. Balasubramanian, G. Ceder, Nature 2018, 556, 185.
- 151
- 151aL. Zhang, H. Noguchi, D. Li, T. Muta, X. Wang, M. Yoshio, I. Taniguchi, J. Power Sources 2008, 185, 534–541;
- 151bM. A. Cambaz, B. P. Vinayan, H. Euchner, R. E. Johnsen, A. A. Guda, A. Mazilkin, Y. V. Rusalev, A. L. Trigub, A. Gross, M. Fichtner, ACS Appl. Mater. Interfaces 2018, 10, 21957–21964.
- 152A. Abdellahi, A. Urban, S. Dacek, G. Ceder, Chem. Mater. 2016, 28, 3659–3665.
- 153A. Urban, A. Abdellahi, S. Dacek, N. Artrith, G. Ceder, Phys. Rev. Lett. 2017, 119, 176402.