High iso Aldehyde Selectivity in the Hydroformylation of Short-Chain Alkenes
Dr. Leo Iu
EaStCHEM School of Chemistry, University of St Andrews, Purdie Building, North Haugh, St Andrews, KY16 9ST UK
Search for more papers by this authorDr. José A. Fuentes
EaStCHEM School of Chemistry, University of St Andrews, Purdie Building, North Haugh, St Andrews, KY16 9ST UK
Search for more papers by this authorDr. Mesfin E. Janka
Eastman Chemical Company, 200 South Wilcox Drive, Kingsport, TN, 37660 USA
Search for more papers by this authorDr. Kevin J. Fontenot
Eastman Chemical Company, 200 South Wilcox Drive, Kingsport, TN, 37660 USA
Search for more papers by this authorCorresponding Author
Prof. Matthew L. Clarke
EaStCHEM School of Chemistry, University of St Andrews, Purdie Building, North Haugh, St Andrews, KY16 9ST UK
Search for more papers by this authorDr. Leo Iu
EaStCHEM School of Chemistry, University of St Andrews, Purdie Building, North Haugh, St Andrews, KY16 9ST UK
Search for more papers by this authorDr. José A. Fuentes
EaStCHEM School of Chemistry, University of St Andrews, Purdie Building, North Haugh, St Andrews, KY16 9ST UK
Search for more papers by this authorDr. Mesfin E. Janka
Eastman Chemical Company, 200 South Wilcox Drive, Kingsport, TN, 37660 USA
Search for more papers by this authorDr. Kevin J. Fontenot
Eastman Chemical Company, 200 South Wilcox Drive, Kingsport, TN, 37660 USA
Search for more papers by this authorCorresponding Author
Prof. Matthew L. Clarke
EaStCHEM School of Chemistry, University of St Andrews, Purdie Building, North Haugh, St Andrews, KY16 9ST UK
Search for more papers by this authorGraphical Abstract
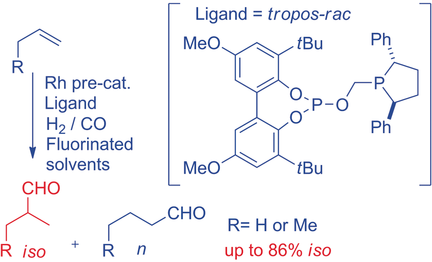
The hydroformylation of propene to give predominantly iso-butanal has been achieved; class-leading selectivity is possible even at higher temperatures that deliver fast conversion. Racemic rhodium complexes of bidentate phospholane phosphites derived from tropos-biphenols and unusual solvent systems are the key to the selectivity observed.
Abstract
The hydroformylation of propene to give predominantly iso-butanal has been achieved; class-leading selectivity is possible even at higher temperatures that deliver fast conversion. Racemic rhodium complexes of bidentate phospholane phosphites derived from tropos-biphenols and unusual solvent systems are the key to the selectivity observed.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201811888-sup-0001-misc_information.pdf2.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aP. W. N. M. van Leeuwen in Rhodium Catalyzed Hydroformylation, Vol. 22 (Eds.: ), Springer Netherlands, Dordrecht, 2002, pp. 1–13;
- 1bR. Franke, D. Selent, A. Börner, Chem. Rev. 2012, 112, 5675–5732;
- 1cA. Börner, R. Franke in Hydroformylation. Fundamentals, Processes, and Applications in Organic Synthesis, Wiley-VCH, Weinheim, 2016.
10.1002/9783527677931 Google Scholar
- 2
- 2aL. Gonsalvi, A. Guerriero, E. Monflier, F. Hapiot, M. Peruzzini in Hydroformylation for Organic Synthesis, Vol. 342 (Eds.: ), Springer Berlin Heidelberg, Berlin, 2013, pp. 1–47.
- 3
- 3aT. J. P. Devon, G. W. Puckette, T. A. Stavinoha, J. L. Vanderbilt (Eastman Kodak Company), US 4694109 A, 1987;
- 3bT. J. P. Devon, G. W. Puckette, T. A. Stavinoha, J. L. Vanderbilt (Eastman Kodak Company), US 5332846 A, 1994;
- 3cC. P. Casey, E. L. Paulsen, E. W. Beuttenmueller, B. R. Proft, L. M. Petrovich, B. A. Matter, D. R. Powell, J. Am. Chem. Soc. 1997, 119, 11817–11825;
- 3dC. P. Casey, E. L. Paulsen, E. W. Beuttenmueller, B. R. Proft, B. A. Matter, D. R. Powell, J. Am. Chem. Soc. 1999, 121, 63–70.
- 4
- 4aE. Billig, A. G. Abatjoglou, D. R. Bryant (Union Carbide), EP 213639, 1987.
- 5
- 5aL. A. van der Veen, M. D. K. Boele, F. R. Bregman, P. C. J. Kamer, P. W. N. M. van Leeuwen, K. Goubitz, J. Fraanje, H. Schenk, C. Bo, J. Am. Chem. Soc. 1998, 120, 11616–11626, and references therein.
- 6
- 6aI. Ojima, K. Kato, M. Okabe, T. Fuchikami, J. Am. Chem. Soc. 1987, 109, 7714–7720;
- 6bC. J. Cobley, K. Gardner, J. Klosin, C. Praquin, C. Hill, G. T. Whiteker, A. Zanotti-Gerosa, J. L. Petersen, K. A. Abboud, J. Org. Chem. 2004, 69, 4031–4040;
- 6cN. Sakai, S. Mano, K. Nozaki, H. Takaya, J. Am. Chem. Soc. 1993, 115, 7033–7034;
- 6dG. M. Noonan, D. Newton, C. J. Cobley, A. Suárez, A. Pizzano, M. L. Clarke, Adv. Synth. Catal. 2010, 352, 1047–1054;
- 6eZ. Yu, M. S. Eno, A. H. Annis, J. P. Morken, Org. Lett. 2015, 17, 3264–3267;
- 6fF. Shibahara, K. Nozaki, T. Hiyama, J. Am. Chem. Soc. 2003, 125, 8555–8560;
- 6gX. Wang, S. L. Buchwald, J. Org. Chem. 2013, 78, 3429–3433;
- 6hB. F. Perandones, C. Godard, C. Claver in Hydroformylation for Organic Synthesis, Vol. 342 (Eds.: ), Springer Berlin Heidelberg, Berlin, 2013, pp. 79–115.
- 7
- 7aM. Leight Abrams, F. Foarta, C. R. Landis, J. Am. Chem. Soc. 2014, 136, 14583–14588;
- 7bA. H. Hoveyda, D. A. Evans, G. C. Fu, Chem. Rev. 1993, 93, 1307–1370;
- 7cC. U. Grünanger, B. Breit, Angew. Chem. Int. Ed. 2008, 47, 7346–7349; Angew. Chem. 2008, 120, 7456–7459;
- 7dA. D. Worthy, C. L. Joe, T. E. Lightburn, K. L. Tan, J. Am. Chem. Soc. 2010, 132, 14757–14759;
- 7eB. Breit, Acc. Chem. Res. 2003, 36, 264–275;
- 7fP. Dydio, W. I. Dzik, M. Lutz, B. de Bruin, J. N. H. Reek, Angew. Chem. Int. Ed. 2011, 50, 396–400; Angew. Chem. 2011, 123, 416–420;
- 7gC. Schmitz, K. Holthusen, W. Leitner, G. Franciò, ACS Catal. 2016, 6, 1584–1589.
- 8
- 8aR. A. Baber, M. L. Clarke, K. M. Heslop, A. C. Marr, A. G. Orpen, P. G. Pringle, A. Ward, D. E. Zambrano-Williams, Dalton Trans. 2005, 1079–1085;
- 8bE. Zuidema, P. E. Goudriaan, B. H. G. Swennenhuis, P. C. J. Kamer, P. W. N. M. van Leeuwen, M. Lutz, A. L. Spek, Organometallics 2010, 29, 1210–1221;
- 8cR. C. How, M. L. Clarke, R. T. Hembre, J. A. Ponasik, G. S. Tolleson (Eastman Chemical Company), US 9308527 B2, 2016;
- 8dR. C. How, R. Hembre, J. A. Ponasik, G. S. Tolleson, M. L. Clarke, Catal. Sci. Technol. 2016, 6, 118–124;
- 8eR. C. How, P. Dingwall, R. T. Hembre, J. A. Ponasik, G. S. Tolleson, M. L. Clarke, Mol. Catal. 2017, 434, 116–122;
- 8fA. Phanopoulos, K. Nozaki, ACS Catal. 2018, 8, 5799–5809.
- 9
- 9aG. M. Noonan, J. A. Fuentes, C. J. Cobley, M. L. Clarke, Angew. Chem. Int. Ed. 2012, 51, 2477–2480; Angew. Chem. 2012, 124, 2527–2530;
- 9bG. M. Noonan, C. J. Cobley, T. Mahoney, M. L. Clarke, Chem. Commun. 2014, 50, 1475–1477;
- 9cR. Pittaway, J. A. Fuentes, M. L. Clarke, Org. Lett. 2017, 19, 2845–2848;
- 9dP. Dingwall, J. A. Fuentes, L. Crawford, A. M. Z. Slawin, M. Bühl, M. L. Clarke, J. Am. Chem. Soc. 2017, 139, 15921–15932;
- 9e(S,S,S)-BOBPHOS is commercially available from Strem Chemicals.
- 10
- 10aD. W. Norman, J. N. H. Reek, T. R. M.-L. Besset (Eastman Chemical Company), US 8710275 B2, 2014;
- 10bn/iso of 0.66 for oct-1-ene at higher T: T. Besset, D. W. Norman, J. N. H. Reek, Adv. Synth. Catal. 2013, 355, 348–352;
- 10cV. F. Slagt, P. C. J. Kamer, P. W. N. M. van Leeuwen, J. N. H. Reek, J. Am. Chem. Soc. 2004, 126, 1526–1536;
- 10dn/iso of 0.84 at 25 °C, 1.1 at 70 °C: X. Wang, S. Nurttila, W. I. Czik, R. Becker, J. Rodgers, J. N. H. Reek, Chem. Eur. J. 2017, 23, 14769–14777.
- 11Grand View Research, Mar 2016, Isobutanol Market Analysis By Product (Synthetic, Bio-Based), Application (Oil & Gas, Solvents & Coatings, Chemical Intermediates) And Segment Forecasts To 2022, https://www.grandviewresearch.com/industry-analysis/isobutanol-market.
- 12
- 12aK. Aikawa, K. Mikami, Chem. Commun. 2012, 48, 11050–11069;
- 12bP. Oczipka, D. Müller, W. Leitner, G. Franciò, Chem. Sci. 2016, 7, 678–683;
- 12cG. Storch, O. Trapp, Angew. Chem. Int. Ed. 2015, 54, 3580–3586; Angew. Chem. 2015, 127, 3650–3656;
- 12dC. Monti, c. Gennari, U. Piarulli, Chem. Commun. 2005, 5281–5283;
- 12eP. W. N. M. van Leeuwen, P. C. J. Kamer, C. Claver, O. Pàmies, M. Diéguez, Chem. Rev. 2011, 111, 2077–2118;
- 12fG. T. Whiteker, A. M. Harrison, A. H. Abatjoglou, Chem. Commun. 1995, 1805–1806.
- 13
- 13aL. C. Clark, F. Gollan, Science 1966, 152, 1755–1756;
- 13bR. H. Fish, Chem. Eur. J. 1999, 5, 1677–1680;
10.1002/(SICI)1521-3765(19990604)5:6<1677::AID-CHEM1677>3.0.CO;2-X CAS Web of Science® Google Scholar
- 13cI. Colomer, A. E. R. Chamberlain, M. B. Haughey, T. J. Donohoe, Nat. Rev. Chem. 2017, 1, 0088;
- 13dA. Lattanzi, C. De Fusco, A. Russo, A. Poaterb, L. Cavallo, Chem. Commun. 2012, 48, 1650–1652;
- 13eC. Samojłowicz, M. Bieniek, A. Pazio, A. Makal, K. Woźniak, A. Poater, L. Cavallo, J. Wójcik, K. Zdanowski, K. Grela, Chem. Eur. J. 2011, 17, 12981–12993.
- 14D. Prescher, V. E. Platonov, K. V. Dvornikova, J. Schulze, O. I. Osina, G. G. Yakobson, J. Fluorine Chem. 1985, 29, 204.
- 15The research data supporting this publication can be accessed at https://doi.org/10.17630/6ed96377-ab54-4fb3-98c9-d2a5bc4d333e.