Visible-Light-Mediated Metal-Free Difunctionalization of Alkenes with CO2 and Silanes or C(sp3)−H Alkanes
Dr. Jing Hou
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Republic of Singapore
Search for more papers by this authorAloysius Ee
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Republic of Singapore
Search for more papers by this authorHui Cao
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Republic of Singapore
Search for more papers by this authorHan-Wee Ong
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Republic of Singapore
Search for more papers by this authorJin-Hui Xu
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Republic of Singapore
Search for more papers by this authorCorresponding Author
Dr. Jie Wu
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Republic of Singapore
Search for more papers by this authorDr. Jing Hou
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Republic of Singapore
Search for more papers by this authorAloysius Ee
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Republic of Singapore
Search for more papers by this authorHui Cao
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Republic of Singapore
Search for more papers by this authorHan-Wee Ong
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Republic of Singapore
Search for more papers by this authorJin-Hui Xu
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Republic of Singapore
Search for more papers by this authorCorresponding Author
Dr. Jie Wu
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Republic of Singapore
Search for more papers by this authorGraphical Abstract
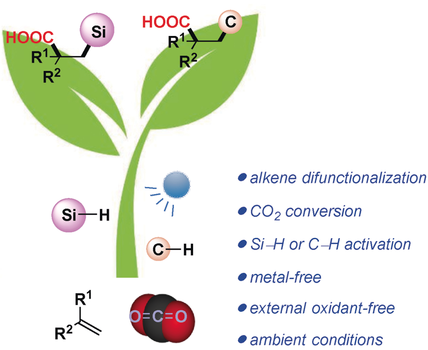
Photocarboxylation: Visible-light-promoted metal-free difunctionalization of alkenes using CO2 and readily available Si−H and C(sp3)−H reagents has been realized by the merging of photoredox and hydrogen-atom-transfer catalysis. A variety of valuable compounds, such as β-silacarboxylic acids and acids bearing a γ-heteroatom (e.g., N, O, S) can be directly accessed from simple alkenes in a redox-neutral fashion.
Abstract
Catalytic alkene difunctionalization via Si−H and C−H activations represents an ideal atom- and step-economic pathway for quick assembly of molecular complexity. We herein developed a visible-light-promoted metal-free difunctionalization of alkenes using abundant CO2 and readily available Si−H and C(sp3)−H bonds as feedstocks. Through the merger of photoredox and hydrogen-atom-transfer catalysis, a variety of value-added compounds, such as β-silacarboxylic acids and acids bearing a γ-heteroatom (e.g., N, O, S) could be directly accessed from simple alkenes in a redox-neutral fashion.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201811266-sup-0001-misc_information.pdf6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews on difunctionalization of alkenes, see:
- 1aJ.-S. Zhang, L. Liu, T. Chen, L.-B. Han, Chem. Asian J. 2018, 13, 2277;
- 1bX.-W. Lan, N.-X. Wang, Y.-L. Xing, Eur. J. Org. Chem. 2017, 5821;
- 1cA. J. McCarroll, J. C. Walton, Angew. Chem. Int. Ed. 2001, 40, 2224;
10.1002/1521-3773(20010618)40:12<2224::AID-ANIE2224>3.0.CO;2-F CAS PubMed Web of Science® Google ScholarAngew. Chem. 2001, 113, 2282.
- 2For selected examples, see:
- 2aA. Bunescu, T. M. Ha, Q. Wang, J. Zhu, Angew. Chem. Int. Ed. 2017, 56, 10555; Angew. Chem. 2017, 129, 10691;
- 2bY. Yang, R.-J. Song, X.-H. Ouyang, C.-Y. Wang, J.-H. Li, S.-L. Luo, Angew. Chem. Int. Ed. 2017, 56, 7916; Angew. Chem. 2017, 129, 8024;
- 2cJ. Zhang, J. Jiang, D. Xu, Q. Luo, H. Wang, J. Chen, H. Li, Y. Wang, X. Wan, Angew. Chem. Int. Ed. 2015, 54, 1231; Angew. Chem. 2015, 127, 1247;
- 2dC. Chatalova-Sazepin, Q. Wang, G. M. Sammis, J. Zhu, Angew. Chem. Int. Ed. 2015, 54, 5443; Angew. Chem. 2015, 127, 5533;
- 2eJ.-K. Cheng, T.-P. Loh, J. Am. Chem. Soc. 2015, 137, 42;
- 2fB. Schweitzer-Chaput, J. Demaerel, H. Engler, M. Klussmann, Angew. Chem. Int. Ed. 2014, 53, 8737; Angew. Chem. 2014, 126, 8882;
- 2gK. Cheng, L. Huang, Y. Zhang, Org. Lett. 2009, 11, 2908.
- 3For selected recent reviews, see:
- 3aL. Marzo, S. K. Pagire, O. Reiser, B. König, Angew. Chem. Int. Ed. 2018, 57, 10034; Angew. Chem. 2018, 130, 10188;
- 3bM. Silvi, P. Melchiorre, Nature 2018, 554, 41;
- 3cN. A. Romero, D. A. Nicewicz, Chem. Rev. 2016, 116, 10075;
- 3dD. Ravelli, S. Protti, M. Fagnoni, Chem. Rev. 2016, 116, 9850;
- 3eK. L. Skubi, T. R. Blum, T. P. Yoon, Chem. Rev. 2016, 116, 10035;
- 3fC. K. Prier, D. A. Rankic, D. W. C. MacMillan, Chem. Rev. 2013, 113, 5322;
- 3gJ. Xuan, W.-J. Xiao, Angew. Chem. Int. Ed. 2012, 51, 6828; Angew. Chem. 2012, 124, 6934;
- 3hJ. M. R. Narayanam, C. R. J. Stephenson, Chem. Soc. Rev. 2011, 40, 102.
- 4
- 4aX. Wu, S. Wu, C. Zhu, Tetrahedron Lett. 2018, 59, 1328;
- 4bM.-Y. Cao, X. Ren, Z. Lu, Tetrahedron Lett. 2015, 56, 3732.
- 5L. Capaldo, D. Ravelli, Eur. J. Org. Chem. 2017, 2056.
- 6
- 6aG. S. Lee, S. H. Hong, Chem. Sci. 2018, 9, 5810;
- 6bJ. S. Li, J. Wu, ChemPhotoChem 2018, 2, 839.
- 7R. Zhou, Y. Y. Goh, H. Liu, H. Tao, L. Li, J. Wu, Angew. Chem. Int. Ed. 2017, 56, 16621; Angew. Chem. 2017, 129, 16848.
- 8For recent reviews, see:
- 8aA. Tortajada, F. Juliá-Hernández, M. Borjesson, T. Moragas, R. Martin, Angew. Chem. Int. Ed. 2018, https://doi.org/10.1002/anie.201803186; Angew. Chem. 2018, https://doi.org/10.1002/ange.201803186;
- 8bJ. Hou, J.-S. Li, J. Wu, Asian J. Org. Chem. 2018, 7, 1439;
- 8cB. Yu, L.-N. He, ChemSusChem 2015, 8, 52;
- 8dN. Kielland, C. J. Whiteoak, A. W. Kleij, Adv. Synth. Catal. 2013, 355, 2115;
- 8eL. Zhang, Z. Hou, Chem. Sci. 2013, 4, 3395.
- 9For selected recent examples, see:
- 9aQ. Y. Meng, S. Wang, G. S. Huff, B. König, J. Am. Chem. Soc. 2018, 140, 3198;
- 9bH. Seo, A. Liu, T. F. Jamison, J. Am. Chem. Soc. 2017, 139, 13969;
- 9cM. Gaydou, T. Moragas, F. Julia-Hernandez, R. Martin, J. Am. Chem. Soc. 2017, 139, 12161;
- 9dK. Murata, N. Numasawa, K. Shimomaki, J. Takaya, N. Iwasawa, Chem. Commun. 2017, 53, 3098;
- 9eY.-Y. Gui, N. Hu, X.-W. Chen, L.-L. Liao, T. Ju, J.-H. Ye, Z. Zhang, J. Li, D.-G. Yu, J. Am. Chem. Soc. 2017, 139, 17011;
- 9fL. Wu, Q. Liu, I. Fleischer, R. Jackstell, M. Beller, Nat. Commun. 2014, 5, 3091.
- 10
- 10aV. R. Yatham, Y. Shen, R. Martin, Angew. Chem. Int. Ed. 2017, 56, 10915; Angew. Chem. 2017, 129, 11055;
- 10bJ.-H. Ye, M. Miao, H. Huang, S.-S. Yan, Z.-B. Yin, W.-J. Zhou, D.-G. Yu, Angew. Chem. Int. Ed. 2017, 56, 15416; Angew. Chem. 2017, 129, 15618;
- 10cT. W. Butcher, E. J. McClain, T. G. Hamilton, T. M. Perrone, K. M. Kroner, G. C. Donohoe, N. G. Akhmedov, J. L. Petersen, B. V. Popp, Org. Lett. 2016, 18, 6428.
- 11
- 11aF. Xue, H. Deng, D. K. B. Mohamed, K. Y. Tang, J. Wu, Chem. Sci. 2017, 8, 3623;
- 11bJ. Hou, A. Ee, W. Feng, J. H. Xu, Y. Zhao, J. Wu, J. Am. Chem. Soc. 2018, 140, 5257;
- 11cH. P. Deng, Q. Zhou, J. Wu, Angew. Chem. Int. Ed. 2018, 57, 12661; Angew. Chem. 2018, 130, 12843.
- 12
- 12aR. Zhou, H. Liu, H. Tao, X. Yu, J. Wu, Chem. Sci. 2017, 8, 4654;
- 12bH. Deng, X. Fan, Z. Chen, Q. Xu, J. Wu, J. Am. Chem. Soc. 2017, 139, 13579;
- 12cX. Fan, J. Rong, H. Wu, Q. Zhou, H. Deng, J. D. Tan, C. Xue, L. Wu, H. Tao, J. Wu, Angew. Chem. Int. Ed. 2018, 57, 8514; Angew. Chem. 2018, 130, 8650.
- 13For recent reports on catalytic silacarboxylation of alkynes and allenes, see:
- 13aT. Fujihara, Y. Tani, K. Semba, J. Terao, Y. Tsuji, Agnew. Chem. Int. Ed. 2012, 51, 11487; Angew. Chem. 2012, 124, 11655;
- 13bY. Tani, T. Fujihara, J. Terao, Y. Tsuji, J. Am. Chem. Soc. 2014, 136, 17706.
- 14See the Supporting Information for more control experiments.
- 15X. Dai, D. Cheng, B. Guan, W. Mao, X. Xu, X. Li, J. Org. Chem. 2014, 79, 7212.
- 16J. Luo, J. Zhang, ACS Catal. 2016, 6, 873.
- 17No silacarboxylation occurred when using simple styrenes or 1,2-disubstituted styrenes as the substrates.
- 18G. K. Min, D. Hernández, T. Skrydstrup, Acc. Chem. Res. 2013, 46, 457. Examples for further transformation of β-silyl acids can be found in the Supporting Information.
- 19M. Ordóñez, C. Cativiela, I. Romero-Estudillo, Tetrahedron: Asymmetry 2016, 27, 999.
- 20D. Cambié, C. Bottecchia, N. J. W. Straathof, V. Hessel, T. Noël, Chem. Rev. 2016, 116, 10276.
- 21M. H. Shaw, V. W. Shurtleff, J. A. Terrett, J. D. Cuthbertson, D. W. C. MacMillan, Science 2016, 352, 1304.
- 22B. A. Sim, D. Griller, D. D. M. Wayner, J. Am. Chem. Soc. 1989, 111, 754.