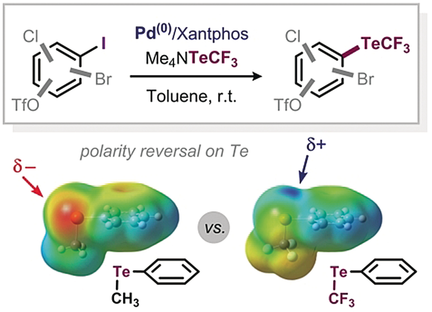
Chemoselective Pd-Catalyzed C-TeCF3 Coupling of Aryl Iodides
Theresa Sperger
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorSinem Guven
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Franziska Schoenebeck
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorTheresa Sperger
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorSinem Guven
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Franziska Schoenebeck
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorGraphical Abstract
Te time: The first direct catalytic method for the formation of C(sp2)−TeCF3 bonds has been developed. It relies on a Pd/Xantphos catalytic system and allows for the trifluoromethylellurolation of aryl iodides. Computational and experimental mechanistic analyses shed light on the privileged activity of Xantphos in this transformation.
Abstract
While the TeCF3 moiety features promising properties and potential in a range of applications, no direct synthetic method exists for its incorporation into aromatic scaffolds. This report features the first direct catalytic method for the formation of C(sp2)−TeCF3 bonds. The method relies on a Pd/Xantphos catalytic system and allows for the trifluoromethyltellurolation of aryl iodides. Our computational and experimental mechanistic analyses shed light on the privileged activity of Xantphos in this transformation.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201810950-sup-0001-misc_information.pdf3.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aL. A. Ba, M. Doring, V. Jamier, C. Jacob, Org. Biomol. Chem. 2010, 8, 4203–4216;
- 1bC. W. Nogueira, G. Zeni, J. B. T. Rocha, Chem. Rev. 2004, 104, 6255–6286.
- 2S. Shaaban, F. Sasse, T. Burkholz, C. Jacob, Bioorg. Med. Chem. 2014, 22, 3610–3619.
- 3
- 3aJ.-f. Poon, V. P. Singh, J. Yan, L. Engman, Chem. Eur. J. 2015, 21, 2447–2457;
- 3bS. F. Fonseca, D. B. Lima, D. Alves, R. G. Jacob, G. Perin, E. J. Lenardao, L. Savegnago, New J. Chem. 2015, 39, 3043–3050;
- 3cP. C. Nobre, E. L. Borges, C. M. Silva, A. M. Casaril, D. M. Martinez, E. J. Lenardão, D. Alves, L. Savegnago, G. Perin, Bioorg. Med. Chem. 2014, 22, 6242–6249;
- 3dR. Amorati, G. F. Pedulli, L. Valgimigli, H. Johansson, L. Engman, Org. Lett. 2010, 12, 2326–2329;
- 3eS. Kumar, H. Johansson, T. Kanda, L. Engman, T. Müller, H. Bergenudd, M. Jonsson, G. F. Pedulli, R. Amorati, L. Valgimigli, J. Org. Chem. 2010, 75, 716–725;
- 3fM. Rooseboom, N. P. E. Vermeulen, F. Durgut, J. N. M. Commandeur, Chem. Res. Toxicol. 2002, 15, 1610–1618.
- 4S. Mecklenburg, S. Shaaban, L. A. Ba, T. Burkholz, T. Schneider, B. Diesel, A. K. Kiemer, A. Roseler, K. Becker, J. Reichrath, A. Stark, W. Tilgen, M. Abbas, L. A. Wessjohann, F. Sasse, C. Jacob, Org. Biomol. Chem. 2009, 7, 4753–4762.
- 5P. Du, U. M. Viswanathan, K. Khairan, T. Buric, N. E. B. Saidu, Z. Xu, B. Hanf, I. Bazukyan, A. Trchounian, F. Hannemann, I. Bernhardt, T. Burkholz, B. Diesel, A. K. Kiemer, K.-H. Schafer, M. Montenarh, G. Kirsch, C. Jacob, MedChemComm 2014, 5, 25–31.
- 6Z. Rappoport in Patai's Chemistry of Functional Groups, Vol. 4 (Eds.: ), Wiley, Chichester, 2014.
- 7
- 7aK. Müller, C. Faeh, F. Diederich, Science 2007, 317, 1881–1886;
- 7bS. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev. 2008, 37, 320–330;
- 7cL. E. Zimmer, C. Sparr, R. Gilmour, Angew. Chem. Int. Ed. 2011, 50, 11860–11871; Angew. Chem. 2011, 123, 12062–12074;
- 7dO. A. Tomashenko, V. V. Grushin, Chem. Rev. 2011, 111, 4475–4521;
- 7eT. Liang, C. N. Neumann, T. Ritter, Angew. Chem. Int. Ed. 2013, 52, 8214–8264; Angew. Chem. 2013, 125, 8372–8423.
- 8Electrostatic potential surfaces were calculated at the M06L/def2-TZVP level of theory based on geometries optimized at ωB97XD/6-31G(d)(LANL2DZ for Te).
- 9L. Vogel, P. Wonner, S. M. Huber, Angew. Chem. Int. Ed. 2018, https://doi.org/10.1002/anie.201809432; Angew. Chem. 2018, https://doi.org/10.1002/ange.201809432.
- 10Alternatively, and of less generality, safety, or functional group tolerance, symmetrical TeR2 compounds can be exchanged with Te(CF3)2 to yield RTeCF3, see:
- 10aD. W. Brown, R. U. Kirss, D. Gordon (Advanced Technology Materials Inc.), US005312983A, 1994. For syntheses of Te(CF3)2, see:
- 10bS. Herberg, D. Naumann, Z. Anorg. Allg. Chem. 1982, 492, 95–102;
- 10cR. Kasemann, C. Lichenheim, G. Nowicki, D. Naumann, Z. Anorg. Allg. Chem. 1995, 621, 213–217;
- 10dE. A. Ganja, C. D. Ontiveros, J. A. Morrison, Inorg. Chem. 1988, 27, 4535–4538.
- 11T. Umemoto, S. Ishihara, J. Am. Chem. Soc. 1993, 115, 2156–2164.
- 12E. Pietrasiak, A. Togni, Organometallics 2017, 36, 3750–3757.
- 13
- 13aA. B. Dürr, H. C. Fisher, I. Kalvet, K. N. Truong, F. Schoenebeck, Angew. Chem. Int. Ed. 2017, 56, 13431–13435; Angew. Chem. 2017, 129, 13616–13620;
- 13bJ.-B. Han, T. Dong, D. A. Vicic, C.-P. Zhang, Org. Lett. 2017, 19, 3919–3922;
- 13cA. B. Dürr, G. Yin, I. Kalvet, F. Napoly, F. Schoenebeck, Chem. Sci. 2016, 7, 1076–1081;
- 13dM. Aufiero, T. Sperger, A. S. Tsang, F. Schoenebeck, Angew. Chem. Int. Ed. 2015, 54, 10322–10326; Angew. Chem. 2015, 127, 10462–10466;
- 13eG. Yin, I. Kalvet, F. Schoenebeck, Angew. Chem. Int. Ed. 2015, 54, 6809–6813; Angew. Chem. 2015, 127, 6913–6917;
- 13fG. Yin, I. Kalvet, U. Englert, F. Schoenebeck, J. Am. Chem. Soc. 2015, 137, 4164–4172;
- 13gC.-P. Zhang, D. A. Vicic, J. Am. Chem. Soc. 2012, 134, 183–185;
- 13hG. Teverovskiy, D. S. Surry, S. L. Buchwald, Angew. Chem. Int. Ed. 2011, 50, 7312–7314; Angew. Chem. 2011, 123, 7450–7452.
- 14For precedence of aryl–chalcogen bond cleavage under Pd-catalysis, see:
- 14aL.-B. Han, N. Choi, M. Tanaka, J. Am. Chem. Soc. 1997, 119, 1795–1796;
- 14bH. J. Gysling, Coord. Chem. Rev. 1982, 42, 133–244.
- 15For an analogous scenario involving SCF3 and SeCF3, see Ref. [13a,c].
- 16Tetramethylammonium trifluoromethyltellurolate was first synthesized by Tyrra et al.: W. Tyrra, N. V. Kirij, D. Naumann, Y. L. Yagupolskii, J. Fluorine Chem. 2004, 125, 1437–1440. We employed a modified procedure based on Tyrra's initial report (see the Supporting Information for details).
- 17J. F. Hartwig in Organotransition Metal Chemistry—From Bonding to Catalysis, University Science Books, Sausalito, 2010, pp. 321–348.
- 18Calculations were performed at the CPCM (toluene) M06L/def2-TZVP//ωB97XD/6-31G(d)(LANL2DZ for Pd, I, Te) level of theory using Gaussian 09 (Revision D.01), M. J. Frisch et al. (see Supporting Information for full reference). For appropriateness of method, see: T. Sperger, I. A. Sanhueza, I. Kalvet, F. Schoenebeck, Chem. Rev. 2015, 115, 9532.
- 19For reviews on the influence of bite angle on reactivity, see:
- 19aP. W. N. M. van Leeuwen, P. C. J. Kamer, J. N. H. Reek, P. Dierkes, Chem. Rev. 2000, 100, 2741–2770;
- 19bP. Dierkes, P. W. N. M. van Leeuwen, J. Chem. Soc. Dalton Trans. 1999, 1519–1530.
- 20Notably, we found that ArTeCF3 compounds were in general very volatile and even heavier compounds sublimed under high vacuum.
- 21Although the reactions presented herein have been performed using 2.5 mol % catalyst (for more convenient weighing), the reaction is equally efficient also at lower catalyst loadings of 0.5 mol %.
- 22For successes in the selective coupling of C–Br vs. C–Cl vs. C–OTf, see:
- 22aG. Espino, A. Kurbangalieva, J. M. Brown, Chem. Commun. 2007, 1742–1744;
- 22bI. Kalvet, G. Magnin, F. Schoenebeck, Angew. Chem. Int. Ed. 2017, 56, 1581–1585; Angew. Chem. 2017, 129, 1603–1607;
- 22cI. Kalvet, T. Sperger, T. Scattolin, G. Magnin, F. Schoenebeck, Angew. Chem. Int. Ed. 2017, 56, 7078–7082; Angew. Chem. 2017, 129, 7184–7188;
- 22dT. Scattolin, E. Senol, G. Yin, Q. Guo, F. Schoenebeck, Angew. Chem. Int. Ed. 2018, 57, 12425–12429;
- 22eS. T. Keaveney, G. Kundu, F. Schoenebeck, Angew. Chem. Int. Ed. 2018, 57, 12573–12577; Angew. Chem. 2018, 130, 12753–12757.
- 23Using Ni-catalysis, C–I selective alkylation over C–Br was seen, see: Ref. [13a], K. Lin, R. J. Wiles, C. B. Kelly, G. H. M. Davies, G. A. Molander, ACS Catal. 2017, 7, 5129–5133.
- 24F. Eckert, A. Klamt, COSMOtherm, Version C3.0, Release 17.01, 2016, COSMOlogic GmbH & Co. KG, Leverkusen (Germany), (http://www.cosmologic.de).
- 25A decomposition reaction furnishing diaryltellurides Ar-Te-Ar and RTe(CF3)F2 (R=Me or aryl) may occur. This decomposition occurs more readily for electron-poor aryls, but can be avoided by minimizing exposure to air by employing flash column chromatography and directly isolating the product (i.e. prompt removal of solvent). For details, please refer to the Supporting Information.