Mechanochemistry of Gaseous Reactants
Corresponding Author
Prof. Dr. Carsten Bolm
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorCorresponding Author
Dr. José G. Hernández
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Carsten Bolm
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorCorresponding Author
Dr. José G. Hernández
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorGraphical Abstract
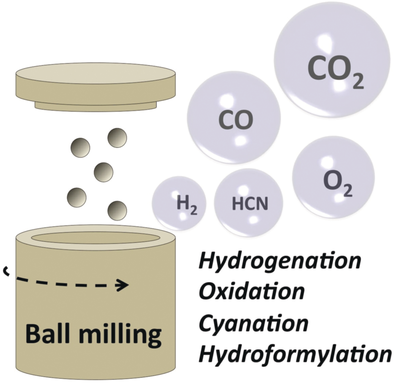
Keep all the balls in the air: This Minireview presents recent examples in the area of mechanochemistry, in which the exertion of mechanical forces by ball milling has enabled chemical transformations with gaseous reactants, together with the technological advances that facilitated such developments.
Abstract
In recent years, the application of mechanical energy to chemical systems has repeatedly proven beneficial to facilitate chemical transformations in various areas in chemistry. Today, a systematic body of evidence indicates that mechanochemistry holds great promise to become a game-changer in chemical synthesis. Not only does mechanochemistry permit access to products that are inaccessible by established means (e.g. purely thermal activation), mechanochemical reactions often outperform their solution-based counterparts in terms of sustainability. Most mechanochemical reactions carried out by ball milling techniques involve transformations of solids and liquids, but the number of mechanochemical reactions with gaseous reactants is increasing. The aim of this Minireview is to provide an overview of recent chemical reactions involving gaseous samples by ball milling techniques and to highlight advances in ball milling technology for the safe handling of gaseous reagents. Examples of reactions proceeding at the interface of solid–/liquid–/gas–gas systems that led to significant improvements in reactivity or selectivity will also be highlighted.
Conflict of interest
The authors declare no conflict of interest.
References
- 1H. Yan, F. Yang, D. Pan, Y. Lin, J. N. Hohman, D. Solis-Ibarra, F. H. Li, J. E. P. Dahl, R. M. K. Carlson, B. A. Tkachenko, A. A. Fokin, P. R. Schreiner, G. Galli, W. L. Mao, Z.-X. Shen, N. A. Melosh, Nature 2018, 554, 505–510.
- 2
- 2aA. L. B. Ramirez, Z. S. Kean, J. A. Orlicki, M. Champhekar, S. M. Elsakr, W. E. Krause, S. L. Craig, Nat. Chem. 2013, 5, 757–761;
- 2bA. Khajeh, X. He, J. Yeon, S. H. Kim, A. Martini, Langmuir 2018, 34, 5971–5977.
- 3S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed, D. C. Waddell, Chem. Soc. Rev. 2012, 41, 413–447.
- 4K. S. Suslick, Faraday Discuss. 2014, 170, 411–422.
- 5
- 5aB. H. Bowser, S. L. Craig, Polym. Chem. 2018, 9, 3583–3593;
- 5bS. Garcia-Manyes, A. E. M. Beedle, Nat. Rev. Chem. 2017, 1, 0083.
- 6
- 6aJ. G. Hernández, Chem. Eur. J. 2017, 23, 17157–17165;
- 6bT. K. Achar, A. Bose, P. Mal, Beilstein J. Org. Chem. 2017, 13, 1907–1931;
- 6cD. Tan, T. Friščić, Eur. J. Org. Chem. 2018, 18–33.
- 7
- 7aP. Baláž, M. Achimovičová, M. Baláž, P. Billik, A. Cherkezova-Zheleva, J. M. Criado, F. Delogu, E. Dutková, E. Gaffet, F. L. Gotor, R. Kumar, I. Mitov, T. Rojac, M. Senna, A. Streletskii, K. Wieczorek-Ciurowa, Chem. Soc. Rev. 2013, 42, 7571–7637;
- 7bE. Boldyreva, Chem. Soc. Rev. 2013, 42, 7719–7738.
- 8N. R. Rightmire, T. P. Hanusa, Dalton Trans. 2016, 45, 2352–2362.
- 9C. Bolm, J. G. Hernández, ChemSusChem 2018, 11, 1410–1420.
- 10
- 10aJ. B. Ravnsbæk, T. M. Swager, ACS Macro Lett. 2014, 3, 305–309;
- 10bS. Grätz, L. Borchardt, RSC Adv. 2016, 6, 64799–64802;
- 10cN. Ohn, J. Shin, S. S. Kim, J. G. Kim, ChemSusChem 2017, 10, 3529–3533;
- 10dK. J. Ardila-Fierro, D. Crawford, A. Körner, S. L. James, C. Bolm, J. G. Hernández, Green Chem. 2018, 20, 1262–1269;
- 10eM. Y. Malca, P.-O. Ferko, T. Friščić, A. Moores, Beilstein J. Org. Chem. 2017, 13, 1963–1968.
- 11L. Takacs, Acta Phys. Pol. A 2012, 121, 711–714.
- 12
- 12aH. C. Erythropel, J. B. Zimmerman, T. M. de Winter, L. Petitjean, F. Melnikov, C. H. Lam, A. W. Lounsbury, K. E. Mellor, N. Z. Janković, Q. Tu, L. N. Pincus, M. M. Falinski, W. Shi, P. Coish, D. L. Plata, P. T. Anastas, Green Chem. 2018, 20, 1929–2961;
- 12bO. Maurin, P. Verdié, G. Subra, F. Lamaty, J. Martinez, T.-X. Métro, Beilstein J. Org. Chem. 2017, 13, 2087–2093.
- 13
- 13aJ. G. Hernández, C. Bolm, J. Org. Chem. 2017, 82, 4007–4019;
- 13bJ.-L. Do, T. Friščić, ACS Cent. Sci. 2017, 3, 13–19.
- 14For selected review articles, see
- 14aG.-W. Wang, Chem. Soc. Rev. 2013, 42, 7668–7700;
- 14bJ. G. Hernández, T. Friščić, Tetrahedron Lett. 2015, 56, 4253–4265;
- 14cJ.-L. Do, T. Friščić, Synlett 2017, 28, 2066–2092;
- 14dA. A. Gečiauskaitė, F. García, Beilstein J. Org. Chem. 2017, 13, 2068–2077;
- 14eM. Leonardi, M. Villacampa, J. C. Menéndez, Chem. Sci. 2018, 9, 2042–2064;
- 14fJ. L. Howard, Q. Cao, D. L. Browne, Chem. Sci. 2018, 9, 3080–3094;
- 14gJ. Andersen, J. Mack, Green Chem. 2018, 20, 1435–1443;
- 14hM. J. Muñoz-Batista, D. Rodriguez-Padron, A. R. Puente-Santiago, R. Luque, ACS Sustainable Chem. Eng. 2018, 6, 9530–9544.
- 15
- 15aD. Margetic, V. Štrukil, Mechanochemical Organic Synthesis, Elsevier, Amsterdam, 2016;
- 15bA. Stolle, B. Ranu, Ball Milling Towards Green Synthesis: Applications, Projects, Challenges, The Royal Society of Chemistry, Cambridge, 2015.
- 16G. N. Hermann, M. t. Unruh, S.-H. Jung, M. Krings, C. Bolm, Angew. Chem. Int. Ed. 2018, 57, 10723–10727; Angew. Chem. 2018, 130, 10883–10887.
- 17D. C. Waddell, I. Thiel, T. D. Clark, S. T. Marcum, J. Mack, Green Chem. 2010, 12, 209–211.
- 18
- 18aN. R. Rightmire, T. P. Hanusa, A. L. Rheingold, Organometallics 2014, 33, 5952–5955;
- 18bA. Garci, K. J. Castor, J. Fakhoury, J.-L. Do, J. Di Trani, P. Chidchob, R. S. Stein, A. K. Mittermaier, T. Friščić, H. Sleiman, J. Am. Chem. Soc. 2017, 139, 16913–16922.
- 19For alternative ball mill configurations often used in chemical synthesis, see
- 19aM. Kessler, R. T. Woodward, N. Wong, R. Rinaldi, ChemSusChem 2018, 11, 552–561;
- 19bL. Chen, B. E. Lemma, J. S. Rich, J. Mack, Green Chem. 2014, 16, 1101–1103;
- 19cS. Dabral, H. Wotruba, J. G. Hernández, C. Bolm, ACS Sustainable Chem. Eng. 2018, 6, 3242–3254.
- 20
- 20aZ.-Y. Zhang, D. Ji, W. Mao, Y. Cui, Q. Wang, L. Han, H. Zhong, Z. Wei, Y. Zhao, K. Nørgaard, T. Li, Angew. Chem. Int. Ed. 2018, 57, 10949–10953; Angew. Chem. 2018, 130, 11115–11119;
- 20bD. W. Peters, R. G. Blair, Faraday Discuss. 2014, 170, 83–91.
- 21P. Baláž, M. Achimovičová, M. Baláž, P. Billik, Z. Cherkezova-Zheleva, J. M. Criado, F. Delogu, E. Dutková, E. Gaffet, F. J. Gotor, R. Kumar, I. Mitov, T. Rojac, M. Senna, A. Streletskii, K. Wieczorek-Ciurowa, Chem. Soc. Rev. 2013, 42, 7571–7637.
- 22For recent studies on temperature development upon ball milling, see
- 22aH. Kulla, S. Haferkamp, I. Akhmetova, M. Rölling, C. Maierhofer, K. Radenmann, F. Emmerling, Angew. Chem. Int. Ed. 2018, 57, 5930–5933; Angew. Chem. 2018, 130, 6034–6038;
- 22bK. Užarević, N. Ferdelji, T. Mrla, P. A. Julien, B. Halasz, T. Friščić, I. Halasz, Chem. Sci. 2018, 9, 2525–2532;
- 22cH. Kulla, M. Wilke, F. Fischer, M. Rölling, C. Maierhofer, F. Emmerling, Chem. Commun. 2017, 53, 1664–1667;
- 22dB. P. Hutchings, D. E. Crawford, L. Gao, P. Hu, S. L. James, Angew. Chem. Int. Ed. 2017, 56, 15252–15256; Angew. Chem. 2017, 129, 15454–15458;
- 22eJ. Andersen, J. Mack, Chem. Sci. 2017, 8, 5447–5453;
- 22fR. Schmidt, H. M. Scholze, A. Stolle, Int. J. Ind. Chem. 2016, 7, 181–186;
- 22gJ. Andersen, J. Mack, Angew. Chem. Int. Ed. 2018, 57, 13062–13065; Angew. Chem. 2018, 130, 13246–13249.
- 23
- 23aL. Takacs, J. Mater. Sci. 2004, 39, 4987–4993;
- 23bL. Takacs, Bull. Hist. Chem. 2003, 28, 26–34;
- 23cL. Takacs, J. Mater. Sci. 2018, 53, 13324–13330;
- 23dL. Takacs, Chem. Soc. Rev. 2013, 42, 7649–7659.
- 24K. Ralphs, C. Hardacre, S. L. James, Chem. Soc. Rev. 2013, 42, 7701–7718.
- 25R. A. Buyanov, V. V. Malchanov, V. V. Boldyrev, Catal. Today 2009, 144, 212–218.
- 26E. Aneggi, V. Rico-Perez, C. de Leitenburg, S. Maschio, L. Soler, J. Llorca, A. Trovarelli, Angew. Chem. Int. Ed. 2015, 54, 14040–14043; Angew. Chem. 2015, 127, 14246–14249.
- 27M. Danielis, S. Colussi, C. de Leitenburg, L. Soler, J. Llorca, A. Trovarelli, Angew. Chem. Int. Ed. 2018, 57, 10212–10216; Angew. Chem. 2018, 130, 10369–10373.
- 28J. F. Yan, W. G. Qiu, L. Y. Song, Y. Chen, Y. C. Su, M. Bai, G. Z. Zhang, H. He, Chem. Commun. 2017, 53, 1321–1324.
- 29W. Zhan, S. Yang, P. Zhang, Y. Guo, G. Lu, M. F. Chisholm, S. Dai, Chem. Mater. 2017, 29, 7323–7329.
- 30S. Mori, W.-C. Xu, T. Ishidzuki, N. Ogasawara, J. Imai, K. Kobayashi, Appl. Catal. A 1996, 137, 255–268.
- 31K. Stangeland, D. Kalai, H. Li, Z. Yu, Energy Procedia 2017, 105, 2022–2027.
- 32G. Mulas, R. Campesi, S. Garroni, F. Delogu, C. Milanese, Appl. Surf. Sci. 2011, 257, 8165–8170.
- 33
- 33aS. Immohr, M. Felderhoff, C. Weidenthaler, F. Schüth, Angew. Chem. Int. Ed. 2013, 52, 12688–12691; Angew. Chem. 2013, 125, 12920–12923;
- 33bS. M. Immohr (2015). Mechanokatalytische Prozesse in der Kugelmühle. PhD thesis. Ruhr-Universität Bochum.
- 34Y. Ren, Z. Ma, L. Qian, S. Dai, H. He, P. G. Bruce, Catal. Lett. 2009, 131, 146–154.
- 35R. Eckert, M. Felderhoff, F. Schüth, Angew. Chem. Int. Ed. 2017, 56, 2445–2448; Angew. Chem. 2017, 129, 2485–2488.
- 36H. Schreyer, S. Immohr, F. Schüth, J. Mater. Sci. 2017, 52, 12021–12030.
- 37A. C. Ferrari, F. Bonaccorso, V. Fal′ko, K. S. Novoselov, S. Roche, P. Bøggild, S. Borini, F. H. L. Koppens, V. Palermo, N. Pugno, J. A. Garrido, R. Sordan, A. Bianco, L. Ballerini, M. Prato, E. Lidorikis, J. Kivioja, C. Marinelli, T. Ryhänen, A. Morpurgo, J. N. Coleman, V. Nicolosi, L. Colombo, A. Fert, M. Garcia-Hernandez, A. Bachtold, G. F. Schneider, F. Guinea, C. Dekker, M. Barbone, Z. Sun, C. Galiotis, A. N. Grigorenko, G. Konstantatos, A. Kis, M. Katsnelson, L. Vandersypen, A. Loiseau, V. Morandi, D. Neumaier, E. Treossi, V. Pellegrini, M. Polini, A. Tredicucci, G. M. Williams, B. H. Hong, J.-H. Ahn, J. M. Kim, H. Zirath, B. J. van Wees, H. van der Zant, L. Occhipinti, A. Di Matteo, I. A. Kinloch, T. Seyller, E. Quesnel, X. Feng, K. Teo, N. Rupesinghe, P. Hakonen, S. R. T. Neil, Q. Tannock, T. Löfwander, J. Kinaret, Nanoscale 2015, 7, 4598–4810.
- 38J. M. González-Domínguez, V. León, M. I. Lucío, M. Prato, E. Vázquez, Nat. Protoc. 2018, 13, 495–506.
- 39V. J. González, A. M. Rodríguez, V. León, J. Frontiñán-Rubio, J. L. Fierro, M. Durán-Prado, A. B. Muñoz-García, M. Pavone, E. Vázquez, Green Chem. 2018, 20, 3581–3592.
- 40I.-Y. Jeon, Y.-R. Shin, G.-J. Sohn, H.-J. Choi, S.-Y. Bae, J. Mahmood, S.-M. Jung, J.-M. Seo, M.-J. Kim, D. Wook Chang, L. Dai, J.-B. Baek, Proc. Natl. Acad. Sci. USA 2012, 109, 5588–5593.
- 41I. Y. Jeon, H. J. Choi, S. M. Jung, J. M. Seo, M. J. Kim, L. Dai, J. B. Baek, J. Am. Chem. Soc. 2013, 135, 1386–1393.
- 42I.-Y. Jeon, H.-J. Choi, M. Choi, J.-M. Seo, S.-M. Jung, M.-J. Kim, S. Zhang, L. Zhang, Z. Xia, L. Dai, N. Park, J.-B. Baek, Sci. Rep. 2013, 3, 1810.
- 43I.-Y. Jeon, M. J. Ju, J. Xu, H.-J. Choi, J.-M. Seo, M.-J. Kim, I. T. Choi, H. M. Kim, J. C. Kim, J.-J. Lee, H. K. Liu, H. K. Kim, S. Dou, L. Dai, J.-B. Baek, Adv. Funct. Mater. 2015, 25, 1170–1179.
- 44J. C. Kim, J.-J. Lee, D. Shin, S.-M. Jung, J.-M. Seo, M.-J. Kim, N. Park, L. Dai, J.-B. Baek, Sci. Rep. 2013, 3, 2260.
- 45D. A. Siddhanti, D. J. Nash, M. A. Navarro, D. M. Mills, A. Khaniya, B. Dhar, W. E. Kaden, K. Y. Chumbimuni-Torres, R. G. Blair, J. Mater. Sci. 2017, 52, 11977–11987.
- 46I.-Y. Jeon, S.-Y. Bae, J.-M. Seo, J.-B. Baek, Adv. Funct. Mater. 2015, 25, 6961–6975.
- 47X. Fan, D. W. Chang, X. Chen, J.-B. Baek, L. Dai, Curr. Opin. Chem. Eng. 2016, 11, 52–58.
- 48A. C. Brezny, C. R. Landis, Acc. Chem. Res. 2018, 51, 2344–2354.
- 49S. S. Nurttila, P. R. Linnebank, T. Krachko, J. N. H. Reek, ACS Catal. 2018, 8, 3469–3488.
- 50K. Cousin, S. Menuel, E. Monflier, F. Hapiot, Angew. Chem. Int. Ed. 2017, 56, 10564–10568; Angew. Chem. 2017, 129, 10700–10704.
- 51For previous examples of mechanochemically made metal complexes and their use in ball mills, see
- 51aC. Schumacher, D. E. Crawford, B. Raguž, R. Glaum, S. L. James, C. Bolm, J. G. Hernández, Chem. Commun. 2018, 54, 8355–8358;
- 51bH. Cheng, J. G. Hernández, C. Bolm, Adv. Synth. Catal. 2018, 360, 1800–1804;
- 51cJ. G. Hernández, C. Bolm, Chem. Commun. 2015, 51, 12582–12584.
- 52F. Meemken, A. Baiker, Chem. Rev. 2017, 117, 11522–11569.
- 53L. T. Alexander, M. G. Byers, J. Chem. Educ. 1932, 9, 916–918.
- 54F. Delogu, Int. J. Hydrogen Energy 2011, 36, 15145–15152.
- 55Y. Sawama, M. Niikawa, Y. Yabe, R. Goto, T. Kawajiri, T. Marumoto, T. Takahashi, M. Itoh, Y. Kimura, Y. Sasai, Y. Yamauchi, S.-i. Kondo, M. Kuzuya, Y. Monguchi, H. Sajiki, ACS Sustainable Chem. Eng. 2015, 3, 683–689.
- 56Y. Sawama, T. Kawajiri, M. Niikawa, R. Goto, Y. Yabe, T. Takahashi, T. Marumoto, M. Itoh, Y. Kimura, Y. Monguchi, S.-I. Kondo, H. Sajiki, ChemSusChem 2015, 8, 3773–3776.
- 57Y. Sawama, N. Yasukawa, K. Ban, R. Goto, M. Niikawa, Y. Monguchi, M. Itoh, H. Sajiki, Org. Lett. 2018, 20, 2892–2896.
- 58C. A. Jaska, K. Temple, A. J. Lough, I. Manners, J. Am. Chem. Soc. 2003, 125, 9424–9434.
- 59Inorganic materials have also been prepared by using dihydrogen in ball mills. For examples, see
- 59aJ. M. Bellosta von Colbe, M. Felderhoff, B. Bogdanović, F. Schüth, C. Weidenthaler, Chem. Commun. 2005, 37, 4732–4734;
- 59bZ. Li, J. Zhang, S. Wang, L. Jiang, M. Latroche, J. Du, F. Cuevas, Phys. Chem. Chem. Phys. 2015, 17, 21927–21934.
- 60D. J. Nash, D. T. Restrepo, N. S. Parra, K. E. Giesler, R. A. Penabade, M. Aminpour, D. Le, Z. Li, O. K. Farha, J. K. Harper, T. S. Rahman, R. G. Blair, ACS Omega 2016, 1, 1343–1354.
- 61S. Torii, K. Jimura, S. Hayashi, R. Kikuchi, A. Takagaki, J. Catal. 2017, 355, 176–184.
- 62S. Das, J. A. Robinson, M. Dubey, H. Terrones, M. Terrones, Annu. Rev. Mater. Res. 2015, 45, 1–27.
- 63A. Y. Li, A. Segalla, C.-J. Li, A. Moores, ACS Sustainable Chem. Eng. 2017, 5, 11752–11760.
- 64A. N. Campbell, S. S. Stahl, Acc. Chem. Res. 2012, 45, 851–863.
- 65R. Schmidt, R. Thorwirth, T. Szuppa, A. Stolle, B. Ondruschka, H. Hopf, Chem. Eur. J. 2011, 17, 8129–8138.
- 66
- 66aG. N. Hermann, P. Becker, C. Bolm, Angew. Chem. Int. Ed. 2015, 54, 7414–7417; Angew. Chem. 2015, 127, 7522–7525;
- 66bfor a similar procedure providing indoles, see G. N. Hermann, C. L. Jung, C. Bolm, Green Chem. 2017, 19, 2520–2523.
- 67A. Beillard, T.-X. Métro, X. Bantreil, J. Martinez, F. Lamaty, Chem. Sci. 2017, 8, 1086–1089.
- 68J. G. Hernández, Beilstein J. Org. Chem. 2017, 13, 1463–1469.
- 69M. Obst, B. König, Beilstein J. Org. Chem. 2016, 12, 2358–2363.
- 70V. Štrukil, I. Sajko, Chem. Commun. 2017, 53, 9101–9104.
- 71A. N. Sokolov, D.-K. Bučar, J. Baltrusaitis, S. X. Gu, L. R. MacGillivray, Angew. Chem. Int. Ed. 2010, 49, 4273–4277; Angew. Chem. 2010, 122, 4369–4373.
- 72E. Colacino, A. Porcheddu, I. Halasz, C. Charnay, F. Delogu, R. Guerra, J. Fullenwarth, Green Chem. 2018, 20, 2973–2977.
- 73N. Mukherjee, T. Chatterjee, B. C. Ranu, J. Org. Chem. 2013, 78, 11110–11114.
- 74T.-X. Métro, J. Martinez, F. Lamaty, ACS Sustainable Chem. Eng. 2017, 5, 9599–9602.
- 75L. Konnert, B. Reneaud, R. M. de Figueiredo, J.-M. Campagne, F. Lamaty, J. Martinez, E. Colacino, J. Org. Chem. 2014, 79, 10132–10142.
- 76E. Troschke, S. Grätz, T. Lübken, L. Borchardt, Angew. Chem. Int. Ed. 2017, 56, 6859–6863; Angew. Chem. 2017, 129, 6963–6967.
- 77M. Đud, O. V. Magdysyuk, D. Margetić, V. Štrukil, Green Chem. 2016, 18, 2666–2674.
- 78J. Kano, E. Kobayashi, W. Tongamp, F. Saito, J. Alloys Compd. 2008, 464, 337–339.
- 79
- 79aY. Sha, Y. Zhang, E. Xu, Z. Wang, T. Zhu, S. L. Craig, ACS Macro Lett. 2018, 7, 1174–1179;
- 79bM. Di Giannantonio, M. A. Ayer, E. Verde-Sesto, M. Lattuada, C. Weder, K. M. Fromm, Angew. Chem. Int. Ed. 2018, 57, 11445–11450; Angew. Chem. 2018, 130, 11616–11621.
- 80C. Lea, Am. J. Sci. 1893, s3-46, 413–420.
10.2475/ajs.s3-46.276.413 Google Scholar
- 81
- 81aH. A. Larsen, H. G. Drickamer, J. Phys. Chem. 1957, 61, 1249–1252;
- 81bP. G. Sim, E. Whalley, J. Phys. Chem. 1987, 91, 1877–1878.
- 82C. Bolm, R. Mocci, C. Schumacher, M. Turberg, F. Puccetti, J. G. Hernández, Angew. Chem. Int. Ed. 2018, 57, 2423–2426; Angew. Chem. 2018, 130, 2447–2450.
- 83D. C. Waddell, T. D. Clark, J. Mack, Tetrahedron Lett. 2012, 53, 4510–4513.
- 84K. S. Rodygin, G. Werner, F. A. Kucherov, V. P. Ananikov, Chem. Asian J. 2016, 11, 965–976.
- 85For recent reports, see
- 85aM. E. Casco, F. Badaczewski, S. Grätz, A. Tolosa, V. Presser, B. Smarsly, L. Borchardt, Carbon 2018, 139, 325–333;
- 85bQ. Li, Y. Li, L. Wu, C. Yang, X. Cui, Carbon 2018, 136, 248–254;
- 85cK. Zhang, S. Tao, X. Xu, H. Meng, Y. Lu, C. Li, Ind. Eng. Chem. Res. 2018, 57, 6180–6188.
- 86M. Turberg, K. J. Ardila-Fierro, C. Bolm, J. G. Hernández, Angew. Chem. Int. Ed. 2018, 57, 10718–10722; Angew. Chem. 2018, 130, 10878–10882.
- 87
- 87aP. A. Julien, I. Malvestiti, T. Friščić, Beilstein J. Org. Chem. 2017, 13, 2160–2168;
- 87bX. Ma, W. Yuan, S. E. J. Bell, S. L. James, Chem. Commun. 2014, 50, 1585–1587.
- 88
- 88aK. Užarević, I. Halasz, T. Friščić, J. Phys. Chem. Lett. 2015, 6, 4129–4140;
- 88bS. Lukin, T. Stolar, M. Tireli, M. V. Blanco, D. Babić, T. Friščić, K. Užarević, I. Halasz, Chem. Eur. J. 2017, 23, 13941–13949;
- 88cL. Batzdorf, F. Fischer, M. Wilke, K.-J. Wenzel, F. Emmerling, Angew. Chem. Int. Ed. 2015, 54, 1799–1802; Angew. Chem. 2015, 127, 1819–1822.