Machine-Driven Enzymatic Oligosaccharide Synthesis by Using a Peptide Synthesizer
Jiabin Zhang
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
These authors contributed equally to this work.
Search for more papers by this authorCongcong Chen
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
These authors contributed equally to this work.
Search for more papers by this authorMadhusudhan Reddy Gadi
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
Search for more papers by this authorChristopher Gibbons
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
Search for more papers by this authorYuxi Guo
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
Search for more papers by this authorDr. Xuefeng Cao
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
Search for more papers by this authorGarrett Edmunds
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
Search for more papers by this authorShuaishuai Wang
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
Search for more papers by this authorDing Liu
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
Search for more papers by this authorDr. Jin Yu
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
Search for more papers by this authorCorresponding Author
Dr. Liuqing Wen
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Peng G. Wang
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
Search for more papers by this authorJiabin Zhang
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
These authors contributed equally to this work.
Search for more papers by this authorCongcong Chen
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
These authors contributed equally to this work.
Search for more papers by this authorMadhusudhan Reddy Gadi
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
Search for more papers by this authorChristopher Gibbons
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
Search for more papers by this authorYuxi Guo
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
Search for more papers by this authorDr. Xuefeng Cao
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
Search for more papers by this authorGarrett Edmunds
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
Search for more papers by this authorShuaishuai Wang
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
Search for more papers by this authorDing Liu
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
Search for more papers by this authorDr. Jin Yu
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
Search for more papers by this authorCorresponding Author
Dr. Liuqing Wen
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Peng G. Wang
Department of Chemistry, Georgia State University, Atlanta, GA, 30303 USA
Search for more papers by this authorGraphical Abstract
Abstract
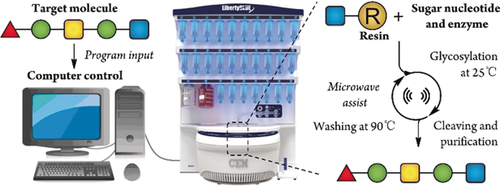
For decades, researchers have endeavored to develop a general automated system to synthesize oligosaccharides that is comparable to the preparation of oligonucleotides and oligopeptides by commercially available machines. Inspired by the success of automated oligosaccharide synthesis through chemical glycosylation, a fully automated system is reported for oligosaccharides synthesis through enzymatic glycosylation in aqueous solution. The designed system is based on the use of a thermosensitive polymer and a commercially available peptide synthesizer. This study represents a proof-of-concept demonstration that the enzymatic synthesis of oligosaccharides can be achieved in an automated manner using a commercially available peptide synthesizer.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201810661-sup-0001-misc_information.pdf2.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1L. Wen, G. Edmunds, C. Gibbons, J. Zhang, M. R. Gadi, H. Zhu, J. Fang, X. Liu, Y. Kong, P. G. Wang, Chem. Rev. 2018, 118, 8151–8187.
- 2C. D. Rillahan, J. C. Paulson, Annu. Rev. Biochem. 2011, 80, 797–823.
- 3Y. Inagaki, P. Song, W. Tang, N. Kokudo, Drug Des. Dev. Ther. 2015, 9, 129–132.
- 4
- 4aL. Wen, M. R. Gadi, Y. Zheng, C. Gibbons, S. M. Kondengaden, J. Zhang, P. G. Wang, ACS Catal. 2018, 8, 7659–7666;
- 4bL. Wen, D. Liu, Y. Zheng, K. Huang, X. Cao, J. Song, P. G. Wang, ACS Cent. Sci. 2018, 4, 451–457;
- 4cL. Wen, Y. Zheng, K. Jiang, M. Zhang, S. M. Kondengaden, S. Li, K. Huang, J. Li, J. Song, P. G. Wang, J. Am. Chem. Soc. 2016, 138, 11473–11476.
- 5Y. L. Huang, C. Y. Wu, Expert Rev. Vaccines 2010, 9, 1257–1274.
- 6R. Das, B. Mukhopadhyay, ChemistryOpen 2016, 5, 401–433.
- 7S. Muthana, H. Z. Cao, X. Chen, Curr. Opin. Chem. Biol. 2009, 13, 573–581.
- 8P. Sears, C. H. Wong, Science 2001, 291, 2344–2350.
- 9
- 9aC. H. Hsu, S. C. Hung, C. Y. Wu, C. H. Wong, Angew. Chem. Int. Ed. 2011, 50, 11872–11923; Angew. Chem. 2011, 123, 12076–12129;
- 9bP. H. Seeberger, Acc. Chem. Res. 2015, 48, 1450–1463.
- 10Z. Y. Zhang, I. R. Ollmann, X. S. Ye, R. Wischnat, T. Baasov, C. H. Wong, J. Am. Chem. Soc. 1999, 121, 734–753.
- 11O. J. Plante, E. R. Palmacci, P. H. Seeberger, Science 2001, 291, 1523–1527.
- 12R. B. Merrifield, J. Am. Chem. Soc. 1963, 85, 2149–2154.
- 13
- 13aO. Calin, S. Eller, P. H. Seeberger, Angew. Chem. Int. Ed. 2013, 52, 5862–5865; Angew. Chem. 2013, 125, 5974–5977;
- 13bH. S. Hahm, M. Hurevich, P. H. Seeberger, Nat. Commun. 2016, 7, 12482;
- 13cS. Eller, M. Collot, J. Yin, H. S. Hahm, P. H. Seeberger, Angew. Chem. Int. Ed. 2013, 52, 5858–5861; Angew. Chem. 2013, 125, 5970–5973;
- 13dL. Kröck, D. Esposito, B. Castagner, C.-C. Wang, P. Bindschädler, P. H. Seeberger, Chem. Sci. 2012, 3, 1617–1622;
- 13eP. H. Seeberger, Chem. Soc. Rev. 2008, 37, 19–28;
- 13fM. Panza, S. G. Pistorio, K. J. Stine, A. V. Demchenko, Chem. Rev. 2018, 118, 8105–8150.
- 14T. Nokami, R. Hayashi, Y. Saigusa, A. Shimizu, C. Y. Liu, K. K. Mong, J. Yoshida, Org. Lett. 2013, 15, 4520–4523.
- 15
- 15aS. G. Pistorio, S. S. Nigudkar, K. J. Stine, A. V. Demchenko, J. Org. Chem. 2016, 81, 8796–8805;
- 15bN. V. Ganesh, K. Fujikawa, Y. H. Tan, K. J. Stine, A. V. Demchenko, Org. Lett. 2012, 14, 3036–3039.
- 16
- 16aK. S. Ko, G. Park, Y. Yu, N. L. Pohl, Org. Lett. 2008, 10, 5381–5384;
- 16bR. Roychoudhury, N. L. Pohl, Org. Lett. 2014, 16, 1156–1159.
- 17
- 17aS. Hanson, M. Best, M. C. Bryan, C. H. Wong, Trends Biochem. Sci. 2004, 29, 656–663;
- 17bX. Chen, P. Kowal, P. G. Wang, Curr. Opin. Drug Discovery Dev. 2000, 3, 756–763.
- 18M. Schuster, P. Wang, J. C. Paulson, C.-H. Wong, J. Am. Chem. Soc. 1994, 116, 1135–1136.
- 19H. Zhu, Z. Wu, M. R. Gadi, S. Wang, Y. Guo, G. Edmunds, W. Guan, J. Fang, Bioorg. Med. Chem. Lett. 2017, 27, 4285–4287.
- 20L. Wen, G. Edmunds, C. Gibbons, J. Zhang, M. R. Gadi, H. Zhu, J. Fang, X. Liu, Y. Kong, P. G. Wang, Chem. Rev. 2018, 118, 8151.
- 21T. Matsushita, I. Nagashima, M. Fumoto, T. Ohta, K. Yamada, H. Shimizu, H. Hinou, K. Naruchi, T. Ito, H. Kondo, S. Nishimura, J. Am. Chem. Soc. 2010, 132, 16651–16656.
- 22X. F. Huang, K. L. Witte, D. E. Bergbreiter, C. H. Wong, Adv. Synth. Catal. 2001, 343, 675–681.
- 23
- 23aR. L. Halcomb, H. Huang, C.-H. Wong, J. Am. Chem. Soc. 1994, 116, 11315–11322;
- 23bM. Meldal, F. I. Auzanneau, O. Hindsgaul, M. M. Palcic, J. Chem. Soc. Chem. Commun. 1994, 1849–1850;
- 23cC. Cai, D. M. Dickinson, L. Li, S. Masuko, M. Suflita, V. Schultz, S. D. Nelson, U. Bhaskar, J. Liu, R. J. Linhardt, Org. Lett. 2014, 16, 2240–2243.
- 24H. Yu, Y. Li, J. Zeng, V. Thon, D. M. Nguyen, T. Ly, H. Y. Kuang, A. Ngo, X. Chen, J. Org. Chem. 2016, 81, 10809–10824.
- 25J. F. Ye, X. W. Liu, P. Peng, W. Yi, X. Chen, F. S. Wang, H. Z. Cao, ACS Catal. 2016, 6, 8140–8144.
- 26B. Sauerzapfe, K. Krenek, J. Schmiedel, W. W. Wakarchuk, H. Pelantova, V. Kren, L. Elling, Glycoconjugate J. 2009, 26, 141–159.
- 27Y. Liu, P. W. Huang, B. M. Jiang, M. Tan, A. L. Morrow, X. Jiang, Plos One 2013, 8, e 78113.
- 28
- 28aK. Lau, V. Thon, H. Yu, L. Ding, Y. Chen, M. M. Muthana, D. Wong, R. Huang, X. Chen, Chem. Commun. 2010, 46, 6066–6068;
- 28bW. Peng, J. Pranskevich, C. Nycholat, M. Gilbert, W. Wakarchuk, J. C. Paulson, N. Razi, Glycobiology 2012, 22, 1453–1464.