Nitromethane as a Carbanion Source for Domino Benzoannulation with Ynones: One-Pot Synthesis of Polyfunctional Naphthalenes and a Total Synthesis of Macarpine
Dr. Shweta Singh
Department of Organic Synthesis and Process Chemistry, CSIR-Indian Institute of Chemical Technology, Tarnaka, Hyderabad-, 500007 India
These authors contributed equally to this work.
Search for more papers by this authorDr. Ramesh Samineni
Department of Organic Synthesis and Process Chemistry, CSIR-Indian Institute of Chemical Technology, Tarnaka, Hyderabad-, 500007 India
Present address: Department of Chemistry, SRMIST, Kattankulathur, Chennai-, 603203 India
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Dr. Srihari Pabbaraja
Department of Organic Synthesis and Process Chemistry, CSIR-Indian Institute of Chemical Technology, Tarnaka, Hyderabad-, 500007 India
Search for more papers by this authorCorresponding Author
Prof. Goverdhan Mehta
School of Chemistry, University of Hyderabad, Hyderabad-, 500046 India
Search for more papers by this authorDr. Shweta Singh
Department of Organic Synthesis and Process Chemistry, CSIR-Indian Institute of Chemical Technology, Tarnaka, Hyderabad-, 500007 India
These authors contributed equally to this work.
Search for more papers by this authorDr. Ramesh Samineni
Department of Organic Synthesis and Process Chemistry, CSIR-Indian Institute of Chemical Technology, Tarnaka, Hyderabad-, 500007 India
Present address: Department of Chemistry, SRMIST, Kattankulathur, Chennai-, 603203 India
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Dr. Srihari Pabbaraja
Department of Organic Synthesis and Process Chemistry, CSIR-Indian Institute of Chemical Technology, Tarnaka, Hyderabad-, 500007 India
Search for more papers by this authorCorresponding Author
Prof. Goverdhan Mehta
School of Chemistry, University of Hyderabad, Hyderabad-, 500046 India
Search for more papers by this authorGraphical Abstract
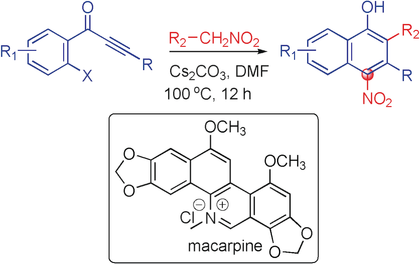
A one-pot, transition-metal-free, domino Michael/SNAr process was devised for the regioselective synthesis of polyfunctional naphthalenes by employing nitromethane and ortho-haloaryl ynones. Nitromethane was utilized as a one-carbon carbanion source that is incorporated into a variety of ynones, ending up as an aromatic nitro substituent. This reaction was used in the total synthesis of the polycyclic alkaloid macarpine.
Abstract
A one-pot, transition-metal-free, domino Michael/SNAr protocol of general applicability has been devised for the regioselective synthesis of polyfunctional naphthalenes by employing nitromethane and ortho-haloaryl ynones. Utilization of nitromethane as a one carbon carbanion source that is incorporated into a variety of ynones, ends up as an aromatic nitro substituent. The application of this domino process towards a total synthesis of the polycyclic alkaloid macarpine demonstrate for the efficacy of this methodology. The conceptually simple approach to affect regioselective, multifunctional benzoannulation of ynones displays wide substrate scope and functional-group tolerance and has been implemented with substituted nitromethanes, as well as with alicyclic o-haloynones.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201810652-sup-0001-misc_information.pdf9.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aN. Abdissa, F. Pan, A. Gruhonjic, J. Grafenstein, P. A. Fitzpatrick, G. Landberg, K. Rissanen, A. Yenesew, M. Erdelyi, J. Nat. Prod. 2016, 79, 2181–2187;
- 1bY. Ge, A. Kazi, F. Marsilio, Y. Luo, S. Jain, W. Brooks, K. G. Daniel, W. C. Guida, S. M. Sebti, H. R. Lawrence, J. Med. Chem. 2012, 55, 1978–1998;
- 1cC. Sun, D. K. Hunt, R. B. Clark, D. Lofland, W. J. O'Brien, L. Plamondon, X.-Y. Xiao, J. Med. Chem. 2011, 54, 3704–3731;
- 1dC. Sun, Q. Wang, J. D. Brubaker, P. M. Wright, C. D. Lerner, K. Noson, M. Charest, D. R. Siegel, Y.-M. Wang, A. G. Myers, J. Am. Chem. Soc. 2008, 130, 17913–17927;
- 1eE. M. Coutinho, Contraception 2002, 65, 259–263;
- 1fT. Ishikawa, T. Saito, H. Ishii, Tetrahedron 1995, 51, 8447–8458;
- 1gP. Sensi, Rev. Infect. Dis. 1983, 5, S 402–S406;
- 1hJ. Slavík, L. Slavikova, Collect. Czech. Chem. Commun. 1955, 20, 356–361.
- 2For reviews, see:
- 2aW. Wu, Y. Liu, D. Zhu, Chem. Soc. Rev. 2010, 39, 1489–1502;
- 2bM. Kertesz, C. H. Choi, S. Yang, Chem. Rev. 2005, 105, 3448–3481;
- 2cM. D. Watson, A. Fechtenkötter, K. Müllen, Chem. Rev. 2001, 101, 1267–1300;
- 2dA. J. Berresheim, M. Muller, K. Mullen, Chem. Rev. 1999, 99, 1747–1786.
- 3For reviews, see:
- 3aS. J. Hein, D. Lehnherr, H. Arslan, F. J. Uribe-Romo, W. R. Dichtel, Acc. Chem. Res. 2017, 50, 2776–2788;
- 3bR. Remy, C. G. Bochet, Chem. Rev. 2016, 116, 9816–9849;
- 3cC. B. de Koning, A. L. Rousseau, W. A. L. van Otterlo, Tetrahedron 2003, 59, 7–36;
- 3dS. Saito, Y. Yamamoto, Chem. Rev. 2000, 100, 2901–2916;
- 3eC. K. Bradsher, Chem. Rev. 1987, 87, 1277–1297.
- 4
- 4aM. L. N. Rao, O. Yamozaki, S. Shimada, T. Tanaka, Y. Suzuki, M. Tanaka, Org. Lett. 2001, 3, 4103–4105;
- 4bD. Zim, V. R. Lando, J. Dupont, A. L. Monteiro, Org. Lett. 2001, 3, 3049–3051;
- 4cD. D. Hennings, T. Iwama, V. H. Rawel, Org. Lett. 1999, 1, 1205–1208.
- 5
- 5aN. Hussain, K. Jana, B. Ganguly, D. Mukherjee, Org. Lett. 2018, 20, 1572–1575;
- 5bJ. Karunakaran, A. K. Mohanakrishnan, Org. Lett. 2018, 20, 966–970.
- 6
- 6aS. Duan, D. K. Sinha-Mahapatra, J. W. Herndon, Org. Lett. 2008, 10, 1541–1544;
- 6bK. H. Dötz, P. Tomuschat, Chem. Soc. Rev. 1999, 28, 187–198.
- 7For selected recent examples, see:
- 7aM. Xiong, H. Hu, X. Hu, Y. Liu, Org. Lett. 2018, 20, 3661–3665;
- 7bF. Wagner, K. Harma, U. Koert, Org. Lett. 2015, 17, 5670–5673;
- 7cG. Naresh, R. Kant, T. Narender, Org. Lett. 2015, 17, 3446–3449;
- 7dH. Y. Kim, K. Oh, Org. Lett. 2014, 16, 5934–5936;
- 7eS. Wang, Z. Chai, Y. Wei, X. Zhu, S. Zhou, S. Wang, Org. Lett. 2014, 16, 3592–3595;
- 7fY. Xia, P. Qu, Z. Liu, R. Ge, Q. Xiao, Y. Zhang, J. Wang, Angew. Chem. Int. Ed. 2013, 52, 2543–2546; Angew. Chem. 2013, 125, 2603–2606;
- 7gP. Gandeepan, C.-H. Cheng, Org. Lett. 2013, 15, 2084–2087;
- 7hD. Kang, J. Kim, S. Oh, P. H. Lee, Org. Lett. 2012, 14, 5636–5639;
- 7iR. Liedtke, M. Harhausen, R. Frohlich, G. Kehr, G. Erker, Org. Lett. 2012, 14, 1448–1451;
- 7jS. Naoe, Y. Suzuki, K. Hirano, Y. Inaba, S. Oishi, N. Fujii, H. Ohno, J. Org. Chem. 2012, 77, 4907–4916;
- 7kC. C. Malakar, D. Schmidt, J. Conrad, U. Beifuss, Org. Lett. 2011, 13, 1972–1975;
- 7lA. R. Jagdale, J. H. Park, S. W. Youn, J. Org. Chem. 2011, 76, 7204–7215.
- 8
- 8aW.-M. Shu, S. Liu, J.-X. He, S. Wang, A.-X. Wu, J. Org. Chem. 2018, 83, 9156–9165;
- 8bK. Okuma, R. Itoyama, A. Sou, N. Nagahora, K. Shioj, Chem. Commun. 2012, 48, 11145–11147;
- 8cW. A. L. van Otterlo, E. L. Ngidi, M. Coyanis, C. B. de Koning, Tetrahedron Lett. 2003, 44, 311–313;
- 8dK.-S. Huang, E.-C. Wang, Tetrahedron Lett. 2001, 42, 6155–6157.
- 9
- 9aH. Ma, X.-Q. Hu, Y.-C. Luo, P.-F. Xu, Org. Lett. 2017, 19, 6666–6669;
- 9bR. A. Novikov, A. V. Tarasova, D. A. Denisov, D. D. Borisov, V. A. Korolev, V. P. Timofeev, Y. V. Tomilov, J. Org. Chem. 2017, 82, 2724–2738;
- 9cA. C. Glass, B. B. Morris, L. N. Zakharov, S.-Y. Liu, Org. Lett. 2008, 10, 4855–4857.
- 10
- 10aH. Liu, L. Ma, R. Zhou, X. Chen, W. Fang, J. Wu, ACS Catal. 2018, 8, 6224–6229;
- 10bJ.-H. Ho, T.-I. Ho, R. S. H. Liu, Org. Lett. 2001, 3, 409–411.
- 11
- 11aC.-K. Chan, Y.-H. Chen, Y.-L. Tsai, M.-Y. Chang, J. Org. Chem. 2017, 82, 3317–3326;
- 11bE. T. Akin, M. Erdogan, A. Dastan, N. Saracoglu, Tetrahedron 2017, 73, 5537–5546;
- 11cJ.-Y. Wang, P. Zhou, G. Li, W.-J. Hao, S.-J. Tu, B. Jiang, Org. Lett. 2017, 19, 6682–6685;
- 11dN. Koppanathi, K. C. Kumara Swamy, Org. Biomol. Chem. 2016, 14, 5079–5087;
- 11eX. Yu, P. Zhu, M. Bao, Y. Yamamoto, A. L. Almansour, N. Arumugam, R. S. Kumar, Asian J. Org. Chem. 2016, 5, 699–704;
- 11fS. Manojveer, R. Balamurugan, Org. Lett. 2014, 16, 1712–1715;
- 11gD. Basavaiah, D. M. Reddy, RSC Adv. 2014, 4, 23966–23970;
- 11hJ.-G. Wang, M. Wang, J.-C. Xiang, Y.-P. Zhu, W.-J. Xue, A.-X. Wu, Org. Lett. 2012, 14, 6060–6063.
- 12L. F. Tietze, Chem. Rev. 1996, 96, 115–136.
- 13
- 13aB. Alcaide, P. Almendros, C. Lazaro-Milla, P. Delgado-Martinez, Chem. Eur. J. 2018, 24, 8186–8194;
- 13bQ. Yao, L. Kong, M. Wang, Y. Yuan, R. Sun, Y. Li, Org. Lett. 2018, 20, 1744–1747;
- 13cR. Samineni, J. Madapa, P. Srihari, G. Mehta, Org. Lett. 2017, 19, 6152–6155;
- 13dF. Zhang, Q. Yao, Y. Yuan, M. Xu, L. Kong, Y. Li, Org. Biomol. Chem. 2017, 15, 2497–2500;
- 13eY. Zhou, X. Tao, Q. Yao, Y. Zhao, Y. Li, Chem. Eur. J. 2016, 22, 17936–17939;
- 13fX. Cheng, Y. Zhou, F. Zhang, K. Zhu, Y. Liu, Y. Li, Chem. Eur. J. 2016, 22, 12655–12659.
- 14A. Y. Sukhorukov, A. A. Sukhanova, S. G. Zlotin, Tetrahedron 2016, 72, 6191–6281.
- 15
- 15aJ. I. G. Cadogan, M. Carmeron-Wood, R. K. Makie, R. J. G. Searle, J. Chem. Soc. 1965, 4831–4837;
- 15bJ. I. G. Cadogan, Organophosphorus Reagents in Organic Synthesis, Academic Press, London, 1979, p. 269.
- 16T. V. Pratap, S. Baskaran, Tetrahedron Lett. 2001, 42, 1983–1985.
- 17CCDC 1841111 (9 a), 1849823 (16 a), 1849824 (16 e), and 1840954 (23) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.