Functionalization of Carbon Monoxide and tert-Butyl Nitrile by Intramolecular Proton Transfer in a Bis(Phosphido) Thorium Complex
Dr. Sean P. Vilanova
Department of Chemistry, University of Missouri, Columbia, MO, 65211 USA
Search for more papers by this authorDr. Iker del Rosal
Universite de Toulouse, CNRS, INSA, UPS, CNRS, UMR, UMR 5215, LPCNO, 135 Avenue de Rangueil, 31077 Toulouse, France
Search for more papers by this authorMichael L. Tarlton
Department of Chemistry, University of Missouri, Columbia, MO, 65211 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Laurent Maron
Universite de Toulouse, CNRS, INSA, UPS, CNRS, UMR, UMR 5215, LPCNO, 135 Avenue de Rangueil, 31077 Toulouse, France
Search for more papers by this authorCorresponding Author
Dr. Justin R. Walensky
Department of Chemistry, University of Missouri, Columbia, MO, 65211 USA
Search for more papers by this authorDr. Sean P. Vilanova
Department of Chemistry, University of Missouri, Columbia, MO, 65211 USA
Search for more papers by this authorDr. Iker del Rosal
Universite de Toulouse, CNRS, INSA, UPS, CNRS, UMR, UMR 5215, LPCNO, 135 Avenue de Rangueil, 31077 Toulouse, France
Search for more papers by this authorMichael L. Tarlton
Department of Chemistry, University of Missouri, Columbia, MO, 65211 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Laurent Maron
Universite de Toulouse, CNRS, INSA, UPS, CNRS, UMR, UMR 5215, LPCNO, 135 Avenue de Rangueil, 31077 Toulouse, France
Search for more papers by this authorCorresponding Author
Dr. Justin R. Walensky
Department of Chemistry, University of Missouri, Columbia, MO, 65211 USA
Search for more papers by this authorGraphical Abstract
Abstract
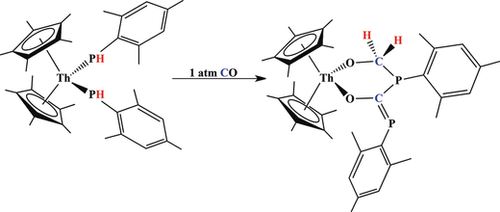
We report intramolecular proton transfer reactions to functionalize carbon monoxide and tert-butyl nitrile from a bis(phosphido) thorium complex. The reaction of (C5Me5)2Th[PH(Mes)]2, Mes=2,4,6-Me3C6H2, with 1 atm of CO yields (C5Me5)2Th(κ2-(O,O)-OCH2PMes-C(O)PMes), in which one CO molecule is inserted into each thorium–phosphorus bond. Concomitant transfer of two protons, formerly coordinated to phosphorus, are now bound to one of the carbon atoms from one of the inserted CO molecules. DFT calculations were employed to determine the lowest energy pathway. With tert-butyl nitrile, tBuCN, only one nitrile inserts into a thorium–phosphorus bond, but the proton is transferred to nitrogen with one phosphido remaining unperturbed affording (C5Me5)2Th[PH(Mes)][κ2-(P,N)-N(H)C(CMe3)P(Mes)]. Surprisingly, reaction of this compound with KN(SiMe3)2 removes the proton bound to nitrogen, not phosphorus.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201810511-sup-0001-misc_information.pdf2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. Zheng, Y. Apeloig, R. Hoffmann, J. Am. Chem. Soc. 1988, 110, 749–774.
- 2H. Schulz, Appl. Catal. A 1999, 186, 3–12.
- 3L. Andrews, B. Liang, J. Li, B. E. Bursten, J. Am. Chem. Soc. 2003, 125, 3126–3139.
- 4J. Li, B. E. Bursten, M. Zhou, L. Andrews, Inorg. Chem. 2001, 40, 5448–5460.
- 5M. Zhou, L. Andrews, J. Li, B. E. Bursten, J. Am. Chem. Soc. 1999, 121, 9712–9721.
- 6M. del Mar Conejo, J. S. Parry, E. Carmona, M. Schultz, J. G. Brennan, S. M. Beshouri, R. A. Anderson, R. D. Rodgers, S. Coles, M. B. Hursthouse, Chem. Eur. J. 1999, 5, 3000–3009.
10.1002/(SICI)1521-3765(19991001)5:10<3000::AID-CHEM3000>3.0.CO;2-Q CAS Web of Science® Google Scholar
- 7J. Parry, E. Carmona, S. Coles, M. Hursthouse, J. Am. Chem. Soc. 1995, 117, 2649–2650.
- 8W. J. Evans, S. A. Kozimor, G. W. Nyce, J. W. Ziller, J. Am. Chem. Soc. 2003, 125, 13831–13835.
- 9R. R. Langeslay, G. P. Chen, C. J. Windorff, A. K. Chan, J. W. Ziller, F. Furche, W. J. Evans, J. Am. Chem. Soc. 2017, 139, 3387–3398.
- 10P. L. Arnold, Chem. Commun. 2011, 47, 9005–9010.
- 11P. L. Arnold, Z. R. Turner, Nat. Rev. Chem. 2017, 1, 0002.
- 12O. T. Summerscales, F. G. N. Cloke, P. B. Hitchcock, J. C. Green, N. Hazari, Science 2006, 311, 829–831.
- 13O. T. Summerscales, F. G. N. Cloke, P. B. Hitchcock, J. C. Green, N. Hazari, J. Am. Chem. Soc. 2006, 128, 9602–9603.
- 14A. S. Frey, F. G. N. Cloke, P. B. Hitchcock, I. J. Day, J. C. Green, G. Aitken, J. Am. Chem. Soc. 2008, 130, 13816–13817.
- 15G. Aitken, N. Hazari, A. S. P. Frey, F. G. N. Cloke, O. Summerscales, J. C. Green, Dalton Trans. 2011, 40, 11080–11088.
- 16D. McKay, A. S. P. Frey, J. C. Green, F. G. N. Cloke, L. Maron, Chem. Commun. 2012, 48, 4118–4120.
- 17N. Tsoureas, O. T. Summerscales, F. G. N. Cloke, S. M. Roe, Organometallics 2013, 32, 1353–1362.
- 18R. J. Kahan, J. H. Farnaby, N. Tsoureas, F. G. N. Cloke, P. B. Hitchcock, M. P. Coles, S. M. Roe, C. Wilson, J. Organomet. Chem. 2018, 857, 110–122.
- 19S. M. Mansell, N. Kaltsoyannis, P. L. Arnold, J. Am. Chem. Soc. 2011, 133, 9036–9051.
- 20M. Falcone, L. Chatelain, R. Scopelliti, I. Živković, M. Mazzanti, Nature 2017, 547, 332.
- 21B. Wang, G. Luo, M. Nishiura, Y. Luo, Z. Hou, J. Am. Chem. Soc. 2017, 139, 16967–16973.
- 22W. J. Evans, D. S. Lee, J. W. Ziller, N. Kaltsoyannis, J. Am. Chem. Soc. 2006, 128, 14176–14184.
- 23A. S. P. Frey, F. G. N. Cloke, M. P. Coles, P. B. Hitchcock, Chem. Eur. J. 2010, 16, 9446–9448.
- 24C. E. Kefalidis, A. S. P. Frey, S. M. Roe, F. G. N. Cloke, L. Maron, Dalton Trans. 2014, 43, 11202–11208.
- 25A. S. P. Frey, F. G. N. Cloke, M. P. Coles, L. Maron, T. Davin, Angew. Chem. Int. Ed. 2011, 50, 6881–6883; Angew. Chem. 2011, 123, 7013–7015.
- 26K. G. Moloy, T. J. Marks, V. W. Day, J. Am. Chem. Soc. 1983, 105, 5696–5698.
- 27D. C. Sonnenberger, E. A. Mintz, T. J. Marks, J. Am. Chem. Soc. 1984, 106, 3484–3491.
- 28P. J. Fagan, K. G. Moloy, T. J. Marks, J. Am. Chem. Soc. 1981, 103, 6959–6962.
- 29J. M. Manriquez, P. J. Fagan, T. J. Marks, C. S. Day, V. W. Day, J. Am. Chem. Soc. 1978, 100, 7112–7114.
- 30P. J. Fagan, J. M. Manriquez, T. J. Marks, V. W. Day, S. H. Vollmer, C. S. Day, J. Am. Chem. Soc. 1980, 102, 5393–5396.
- 31D. A. Katahira, K. G. Moloy, T. J. Marks, Organometallics 1982, 1, 1723–1726.
- 32N. S. Settineri, J. Arnold, Chem. Sci. 2018, 9, 2831–2841.
- 33P. L. Arnold, Z. R. Turner, R. M. Bellabarba, R. P. Tooze, Chem. Sci. 2011, 2, 77–79.
- 34B. M. Gardner, J. C. Stewart, A. L. Davis, J. McMaster, W. Lewis, A. J. Blake, S. T. Liddle, Proc. Natl. Acad. Sci. USA 2012, 109, 9265–9270.
- 35R. G. Pearson, J. Am. Chem. Soc. 1963, 85, 3533–3539.
- 36P. G. Edwards, M. B. Hursthouse, K. M. A. Malik, J. S. Parry, Chem. Commun. 1994, 1249–1250.
- 37P. G. Edwards, J. S. Parry, P. W. Read, Organometallics 1995, 14, 3649–3658.
- 38D. M. Roddick, B. D. Santarsiero, J. E. Bercaw, J. Am. Chem. Soc. 1985, 107, 4670–4678.
- 39Y. Lv, C. E. Kefalidis, J. Zhou, L. Maron, X. Leng, Y. Chen, J. Am. Chem. Soc. 2013, 135, 14784–14796.
- 40A. C. Behrle, J. R. Walensky, Dalton Trans. 2016, 45, 10042–10049.
- 41T. van Dijk, S. Burck, M. K. Rong, A. J. Rosenthal, M. Nieger, J. C. Slootweg, K. Lammertsma, Angew. Chem. Int. Ed. 2014, 53, 9068–9071; Angew. Chem. 2014, 126, 9214–9217.
- 42X. Li, H. Song, C. Cui, Dalton Trans. 2009, 9728–9730.
- 43T. van Dijk, M. K. Rong, J. E. Borger, M. Nieger, J. C. Slootweg, K. Lammertsma, Organometallics 2016, 35, 827–835.
- 44P. Karin, N. Martin, N. Edgar, Angew. Chem. Int. Ed. Engl. 1995, 34, 2369–2371; Angew. Chem. 1995, 107, 2600–2602.
- 45T. M. Rookes, E. P. Wildman, G. Balazs, B. M. Gardner, A. J. Wooles, M. Gregson, F. Tuna, M. Scheer, S. T. Liddle, Angew. Chem. Int. Ed. 2018, 57, 1332–1336; Angew. Chem. 2018, 130, 1346–1350.
- 46P. Yang, E. Zhou, G. Hou, G. Zi, W. Ding, M. D. Walter, Chem. Eur. J. 2016, 22, 13845–13849.
- 47W. Ren, G. Zi, M. D. Walter, Organometallics 2012, 31, 672–679.
- 48W. J. Evans, J. R. Walensky, J. W. Ziller, A. L. Rheingold, Organometallics 2009, 28, 3350–3357.
- 49Z. Hou, D. W. Stephan, J. Am. Chem. Soc. 1992, 114, 10088–10089.
- 50S. P. Vilanova, P. Alayoglu, M. Heidarian, P. Huang, J. R. Walensky, Chem. Eur. J. 2017, 23, 16748–16752.
- 51B. M. Gardner, G. Balázs, M. Scheer, F. Tuna, E. J. L. McInnes, J. McMaster, W. Lewis, A. J. Blake, S. T. Liddle, Angew. Chem. Int. Ed. 2014, 53, 4484–4488; Angew. Chem. 2014, 126, 4573–4577.
- 52J. G. Brennan, R. A. Andersen, J. Am. Chem. Soc. 1985, 107, 514–516.
- 53E. J. Schelter, P. Yang, B. L. Scott, R. E. Da Re, K. C. Jantunen, R. L. Martin, P. J. Hay, D. E. Morris, J. L. Kiplinger, J. Am. Chem. Soc. 2007, 129, 5139–5152.
- 54S. P. Vilanova, M. L. Tarlton, C. L. Barnes, J. R. Walensky, J. Organomet. Chem. 2018, 857, 159–163.
- 55A. C. Behrle, L. Castro, L. Maron, J. R. Walensky, J. Am. Chem. Soc. 2015, 137, 14846–14849.
- 56J. M. Berg, D. L. Clark, J. C. Huffman, D. E. Morris, A. P. Sattelberger, W. E. Streib, W. G. Van der Sluys, J. G. Watkin, J. Am. Chem. Soc. 1992, 114, 10811–10821.
- 57L. Castro, A. Yahia, L. Maron, Dalton Trans. 2010, 39, 6682–6692.
- 58P. Rungthanaphatsophon, A. Bathelier, L. Castro, A. C. Behrle, C. L. Barnes, L. Maron, J. R. Walensky, Angew. Chem. Int. Ed. 2017, 56, 12925–12929; Angew. Chem. 2017, 129, 13105–13109.
- 59E. L. Werkema, L. Maron, O. Eisenstein, R. A. Andersen, J. Am. Chem. Soc. 2007, 129, 2529–2541.
- 60A. Yahia, L. Castro, L. Maron, Chem. Eur. J. 2010, 16, 5564–5567.
- 61M. H. V. Huynh, T. J. Meyer, Chem. Rev. 2007, 107, 5004–5064.
- 62D. R. Weinberg, C. J. Gagliardi, J. F. Hull, C. F. Murphy, C. A. Kent, B. C. Westlake, A. Paul, D. H. Ess, D. G. McCafferty, T. J. Meyer, Chem. Rev. 2012, 112, 4016–4093.
- 63C. P. Sindlinger, L. Wesemann, Chem. Sci. 2014, 5, 2739–2746.
- 64C. P. Sindlinger, W. Granheis, F. S. W. Aicher, L. Wesemann, Chem. Eur. J. 2016, 22, 7554–7566.
- 65F. S. W. Aicher, K. Eichele, H. Schubert, L. Wesemann, Organometallics 2018, 37, 1773–1780.
- 66P. T. Wolczanski, J. E. Bercaw, Acc. Chem. Res. 1980, 13, 121–127.
- 67T. Shima, Z. Hou, J. Am. Chem. Soc. 2006, 128, 8124–8125.
- 68J. Cheng, M. J. Ferguson, J. Takats, J. Am. Chem. Soc. 2010, 132, 2–3.
- 69H. Wang, L. Wu, Z. Lin, Z. Xie, Angew. Chem. Int. Ed. 2018, 57, 8708–8713; Angew. Chem. 2018, 130, 8844–8849.
- 70M. M. Deegan, J. C. Peters, J. Am. Chem. Soc. 2017, 139, 2561–2564.
- 71Y. Katsuma, N. Tsukahara, L. Wu, Z. Lin, M. Yamashita, Angew. Chem. Int. Ed. 2018, 57, 6109–6114; Angew. Chem. 2018, 130, 6217–6222.
- 72L. Keweloh, N. Aders, A. Hepp, D. Pleschka, E.-U. Wurthwein, W. Uhl, Dalton Trans. 2018, 47, 8402–8417.
- 73A. J. M. Miller, J. A. Labinger, J. E. Bercaw, J. Am. Chem. Soc. 2010, 132, 3301–3303.
- 74A. J. M. Miller, J. A. Labinger, J. E. Bercaw, Organometallics 2010, 29, 4499–4516.