Digging into the Sequential Space of Thiolactone Precision Polymers: A Combinatorial Strategy to Identify Functional Domains
Sensu Celasun
Laboratory for Organic Synthesis of Functional Systems, Department of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Strassse 2, 12489 Berlin, Germany
Search for more papers by this authorDario Remmler
Laboratory for Organic Synthesis of Functional Systems, Department of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Strassse 2, 12489 Berlin, Germany
Division 1.5 Protein Analysis, Federal Institute for Materials Research and Testing (BAM), Richard-Willstätter-Strasse 11, 12489 Berlin, Germany
Search for more papers by this authorTimm Schwaar
Division 1.5 Protein Analysis, Federal Institute for Materials Research and Testing (BAM), Richard-Willstätter-Strasse 11, 12489 Berlin, Germany
Search for more papers by this authorDr. Michael G. Weller
Division 1.5 Protein Analysis, Federal Institute for Materials Research and Testing (BAM), Richard-Willstätter-Strasse 11, 12489 Berlin, Germany
Search for more papers by this authorProf. Filip Du Prez
Polymer Chemistry Research group, Centre of Macromolecular Chemistry (CMaC), Department of Organic and Macromolecular Chemistry, Ghent University, Krijgslaan 281 S4, 9000 Ghent, Belgium
Search for more papers by this authorCorresponding Author
Prof. Hans G. Börner
Laboratory for Organic Synthesis of Functional Systems, Department of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Strassse 2, 12489 Berlin, Germany
Search for more papers by this authorSensu Celasun
Laboratory for Organic Synthesis of Functional Systems, Department of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Strassse 2, 12489 Berlin, Germany
Search for more papers by this authorDario Remmler
Laboratory for Organic Synthesis of Functional Systems, Department of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Strassse 2, 12489 Berlin, Germany
Division 1.5 Protein Analysis, Federal Institute for Materials Research and Testing (BAM), Richard-Willstätter-Strasse 11, 12489 Berlin, Germany
Search for more papers by this authorTimm Schwaar
Division 1.5 Protein Analysis, Federal Institute for Materials Research and Testing (BAM), Richard-Willstätter-Strasse 11, 12489 Berlin, Germany
Search for more papers by this authorDr. Michael G. Weller
Division 1.5 Protein Analysis, Federal Institute for Materials Research and Testing (BAM), Richard-Willstätter-Strasse 11, 12489 Berlin, Germany
Search for more papers by this authorProf. Filip Du Prez
Polymer Chemistry Research group, Centre of Macromolecular Chemistry (CMaC), Department of Organic and Macromolecular Chemistry, Ghent University, Krijgslaan 281 S4, 9000 Ghent, Belgium
Search for more papers by this authorCorresponding Author
Prof. Hans G. Börner
Laboratory for Organic Synthesis of Functional Systems, Department of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Strassse 2, 12489 Berlin, Germany
Search for more papers by this authorGraphical Abstract
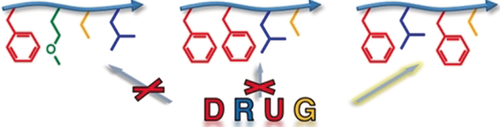
Sequence coins function: Sequences that bind m-THPC are selected from a library of thiolactone (Tla)/Michael oligomers by single-bead readout with MALDI-TOF MS/MS. The corresponding Tla/Michael-PEG conjugates make m-THPC available in solution and allow drug payload as well as drug release kinetics to be fine-tuned by sequence variation in the precision segment.
Abstract
Functional sequences of precision polymers based on thiolactone/Michael chemistry are identified from a large one-bead one-compound library. Single-bead readout by MALDI-TOF MS/MS identifies sequences that host m-THPC that is a second generation photo-sensitizer drug. The corresponding Tla/Michael-PEG conjugates make m-THPC available in solution and drug payload as well as drug release kinetics can be fine-tuned by the precision segment.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201810393-sup-0001-misc_information.pdf3.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. F. Lutz, J. M. Lehn, E. W. Meijer, K. Matyjaszewski, Nat. Rev. Mater. 2016, 1, 16024;
- 1bJ. F. Lutz, M. Ouchi, D. R. Liu, M. Sawamoto, Science 2013, 341, 1238149;
- 1cL. Hartmann, H. G. Borner, Adv. Mater. 2009, 21, 3425–3431;
- 1dM. Ouchi, M. Sawamoto, Polym. J. 2018, 50, 83–94.
- 2
- 2aJ. Lu, C. Fu, S. Wang, L. Tao, L. Yan, D. M. Haddleton, G. Chen, Y. Wei, Macromolecules 2014, 47, 4676–4683;
- 2bH. G. Börner, Macromol. Rapid Commun. 2011, 32, 115–126.
- 3
- 3aI. Cobo, M. Li, B. S. Sumerlin, S. Perrier, Nat. Mater. 2015, 14, 143–159;
- 3bH. G. Börner, Prog. Polym. Sci. 2009, 34, 811–851;
- 3cT. Schnitzler, A. Herrmann, Acc. Chem. Res. 2012, 45, 1419–1430.
- 4
- 4aH. G. Börner, H. Kühnle, J. Hentschel, J. Polym. Sci. Part A 2010, 48, 1–14;
- 4bS. Kessel, A. Thomas, H. G. Börner, Angew. Chem. Int. Ed. 2007, 46, 9023–9026; Angew. Chem. 2007, 119, 9181–9184;
- 4cK. Liu, L. Zheng, C. Ma, R. Gostl, A. Herrmann, Chem. Soc. Rev. 2017, 46, 5147–5172;
- 4dF. Hanßke, E. Kemnitz, G. H. Börner, Small 2015, 11, 4303–4308;
- 4eV. Samsoninkova, B. Seidt, F. Hanßke, W. Wagermaier, H. G. Börner, Adv. Mater. Interfaces 2017, 4, 1600501;
- 4fS. Große, P. Wilke, H. G. Börner, Angew. Chem. Int. Ed. 2016, 55, 11266–11270; Angew. Chem. 2016, 128, 11435–11440;
- 4gP. Wilke, N. Helfricht, A. Mark, G. Papastavrou, D. Faivre, G. H. Börner, J. Am. Chem. Soc. 2014, 136, 12667–12674;
- 4hK. A. Günay, D. L. Berthier, H. A. Jerri, D. Benczedi, H. A. Klok, A. Herrmann, ACS Appl. Mater. Interfaces 2017, 9, 24238–24249.
- 5
- 5aR. N. Zuckermann, J. M. Kerr, S. B. H. Kent, W. H. Moos, J. Am. Chem. Soc. 1992, 114, 10646–10647;
- 5bK. Takizawa, C. Tang, C. J. Hawker, J. Am. Chem. Soc. 2008, 130, 1718–1726;
- 5cM. Porel, C. A. Alabi, J. Am. Chem. Soc. 2014, 136, 13162–13165;
- 5dJ. J. Haven, J. Vandenbergh, R. Kurita, J. Gruber, T. Junkers, Polym. Chem. 2015, 6, 5752–5765;
- 5eS. C. Solleder, D. Zengel, K. S. Wetzel, M. A. Meier, Angew. Chem. Int. Ed. 2016, 55, 1204–1207; Angew. Chem. 2016, 128, 1222–1225.
- 6
- 6aS. Ida, M. Ouchi, M. Sawamoto, J. Am. Chem. Soc. 2010, 132, 14748–14750;
- 6bN. Ten Brummelhuis, Polym. Chem. 2015, 6, 654–667;
- 6cJ. Vandenbergh, G. Reekmans, P. Adriaensens, T. Junkers, Chem. Commun. 2013, 49, 10358–10360;
- 6dJ. Xu, C. Fu, S. Shanmugam, C. J. Hawker, G. Moad, C. Boyer, Angew. Chem. Int. Ed. 2017, 56, 8376–8383; Angew. Chem. 2017, 129, 8496–8503;
- 6eW. R. Gutekunst, C. J. Hawker, J. Am. Chem. Soc. 2015, 137, 8038–8041.
- 7
- 7aJ. Sun, R. N. Zuckermann, ACS Nano 2013, 7, 4715–4732;
- 7bT. T. Trinh, L. Oswald, D. Chan-Seng, J. F. Lutz, Macromol. Rapid Commun. 2014, 35, 141–145;
- 7cR. K. Roy, A. Meszynska, C. Laure, L. Charles, C. Verchin, J. F. Lutz, Nat. Commun. 2015, 6, 7237;
- 7dP. Espeel, L. L. Carrette, K. Bury, S. Capenberghs, J. C. Martins, F. E. Du Prez, A. Madder, Angew. Chem. Int. Ed. 2013, 52, 13261–13264; Angew. Chem. 2013, 125, 13503–13506.
- 8S. Martens, J. Van den Begin, A. Madder, F. E. Du Prez, P. Espeel, J. Am. Chem. Soc. 2016, 138, 14182–14185.
- 9S. Celasun, F. Du Prez, H. G. Börner, Macromol. Rapid Commun. 2017, 38, 1700688.
- 10
- 10aD. Karamessini, T. Simon-Yarza, S. Poyer, E. Konishcheva, L. Charles, D. Letourneur, J.-F. Lutz, Angew. Chem. Int. Ed. 2018, 57, 10574–10578; Angew. Chem. 2018, 130, 10734–10738;
- 10bM. Porel, D. N. Thornlow, N. N. Phan, C. A. Alabi, Nat. Chem. 2016, 8, 590–596;
- 10cA. Al Ouahabi, J. A. Amalian, L. Charles, J. F. Lutz, Nat. Commun. 2017, 8, 967.
- 11S. Wieczorek, E. Krause, S. Hackbarth, B. Röder, A. K. H. Hirsch, G. H. Börner, J. Am. Chem. Soc. 2013, 135, 1711–1714.
- 12
- 12aS. Wieczorek, D. Remmler, T. Masini, Z. Kochovski, A. K. Hirsch, H. G. Börner, Bioconjugate Chem. 2017, 28, 760–767;
- 12bF. Zabihi, S. Wieczorek, M. Dimd, S. Hedtrich, H. G. Börner, R. Haag, J. Controlled Release 2016, 242, 35–41.
- 13
- 13aA. K. H. Hirsch, F. Diederich, M. Antonietti, H. G. Börner, Soft Matter 2010, 6, 88–91;
- 13bS. Wieczorek, A. Dallmann, Z. Kochovski, H. G. Börner, J. Am. Chem. Soc. 2016, 138, 9349–9352;
- 13cC. Lawatscheck, M. Pickhardt, S. Wieczorek, A. Grafmüller, E. Mandelkow, H. G. Börner, Angew. Chem. Int. Ed. 2016, 55, 8752–8756; Angew. Chem. 2016, 128, 8894–8899.
- 14A. W. Purcell, W. Zeng, N. A. Mifsud, L. K. Ely, W. A. Macdonald, D. C. Jackson, J. Pept. Sci. 2003, 9, 255–281.
- 15G. Pasut, Polymers 2014, 6, 160–178.
- 16K. S. Lam, S. E. Salmon, E. M. Hersh, V. J. Hruby, W. M. Kazmierski, R. J. Knapp, Nature 1991, 354, 82.
- 17Software deposit, T. Schwaar, D. Remmler, M. G. Weller, H. G. Börner, Humboldt-Universität zu Berlin, 2018, doi. 10.18452/19202.
- 18D. Remmler, T. Schwaar, M. Pickhardt, C. Donth, E. Mandelkow, M. G. Weller, H. G. Börner, J. Controlled Release 2018, 285, 96–105.