Diazuleno-s-indacene Diradicaloids: Syntheses, Properties, and Local (anti)Aromaticity Shift from Neutral to Dicationic State
Graphical Abstract
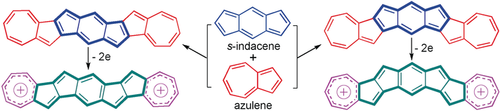
Character building: Derivatives of two diazuleno-s-indacene isomers were synthesized, and they show different diradical character depending on the fusion mode and substituents. They maintain the aromatic character of azulene and anti-aromatic feature of the s-indacene. Upon oxidation to the dication, two aromatic tropylium rings are formed, resulting in a shift of the local (anti)aromaticity.
Abstract
Non-alternant, non-benzenoid π-conjugated polycyclic hydrocarbons (PHs) are expected to exhibit very different electronic properties from all-benzenoid PHs. Reported herein are the syntheses and physical properties of four derivatives of two azulene-fused s-indacene isomers, the diazuleno[2,1-a:2′,1′-g]-s-indacene (DAI-1) and diazuleno[2,1-a:1′,2′-h]-s-indacene (DAI-2). The backbone of both isomers contains 28π electrons and is a 7-5-5-6-5-5-7 fused ring system. X-ray crystallographic analysis, NMR spectra, and theoretical calculations (ACID, NICS) reveal a structure with two aromatic azulene units fused with a central anti-aromatic s-indacene moiety. All compounds exhibit open-shell diradical character and are magnetically active, but the derivatives of DAI-2 show larger radical character than the respective ones of DAI-1. Their dications were isolated in crystalline form and all experimental and theoretical analyses disclose a shift of local (anti)aromaticity along the backbone, with two aromatic tropylium rings at the termini.