Grouping Effect of Single Nickel−N4 Sites in Nitrogen-Doped Carbon Boosts Hydrogen Transfer Coupling of Alcohols and Amines
Hui Su
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P. R. China
Search for more papers by this authorPeng Gao
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P. R. China
Search for more papers by this authorMeng-Ying Wang
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P. R. China
Search for more papers by this authorGuang-Yao Zhai
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P. R. China
Search for more papers by this authorJun-Jun Zhang
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P. R. China
Search for more papers by this authorTian-Jian Zhao
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P. R. China
Search for more papers by this authorJuan Su
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P. R. China
Search for more papers by this authorProf. Markus Antonietti
Department of Colloid Chemistry, Max Planck Institute of Colloids and Interfaces, Potsdam-Golm Science Park, 14476 Potsdam, Germany
Search for more papers by this authorCorresponding Author
Prof. Xin-Hao Li
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Jie-Sheng Chen
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P. R. China
Search for more papers by this authorHui Su
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P. R. China
Search for more papers by this authorPeng Gao
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P. R. China
Search for more papers by this authorMeng-Ying Wang
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P. R. China
Search for more papers by this authorGuang-Yao Zhai
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P. R. China
Search for more papers by this authorJun-Jun Zhang
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P. R. China
Search for more papers by this authorTian-Jian Zhao
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P. R. China
Search for more papers by this authorJuan Su
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P. R. China
Search for more papers by this authorProf. Markus Antonietti
Department of Colloid Chemistry, Max Planck Institute of Colloids and Interfaces, Potsdam-Golm Science Park, 14476 Potsdam, Germany
Search for more papers by this authorCorresponding Author
Prof. Xin-Hao Li
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Jie-Sheng Chen
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P. R. China
Search for more papers by this authorGraphical Abstract
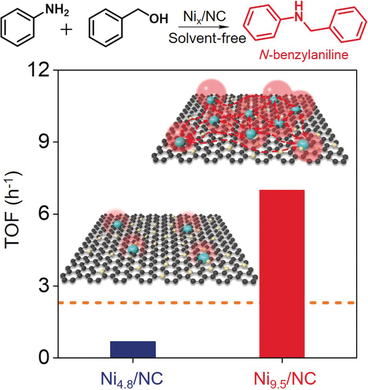
Herd instinct: A grouping effect is observed for single Ni−N4 sites in a Ni catalyst supported by nitrogen-doped carbon (NC). A high 9.5 wt % Ni content was achieved with a ligand-stabilized polycondensation method. An enhanced electron density effect manifests at each single Ni−N4 site, which decreases the binding energy of H radicals to Ni and promotes efficient coupling of benzyl alcohol and aniline to form N-benzylaniline.
Abstract
As a new type of heterogeneous catalyst with “homogeneous-like” activity, single-site transition-metal materials are usually treated as integrated but separate active centers. A novel grouping effect is reported for single Ni−N4 sites in nitrogen-doped carbon (Ni/NC), where an effective ligand-stabilized polycondensation method endows Ni/NC nanocatalysts with a high content of single-site Ni up to 9.5 wt %. The enhanced electron density at each single Ni−N4 site promotes a highly efficient hydrogen transfer, which is exemplified by the coupling of benzyl alcohol and aniline into N-benzylaniline with a turnover frequency (TOF) value of 7.0 molN-benzylaniline molmetal−1 h−1; this TOF outpaces that of reported stable non-noble-metal-based catalysts by a factor of 2.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201809858-sup-0001-misc_information.pdf2.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. J. A. Watson, J. M. J. Williams, Science 2010, 329, 635;
- 1bF. Alonso, P. Riente, M. Yus, Acc. Chem. Res. 2011, 44, 379;
- 1cY. Han, Z. Wang, R. Xu, W. Zhang, W. Chen, L. Zheng, J. Zhang, J. Luo, K. Wu, Y. Zhu, C. Chen, Q. Peng, Q. Liu, P. Hu, D. Wang, Y. Li, Angew. Chem. Int. Ed. 2018, 57, 11262; Angew. Chem. 2018, 130, 11432.
- 2
- 2aY. Zhao, S. W. Foo, S. Saito, Angew. Chem. Int. Ed. 2011, 50, 3006; Angew. Chem. 2011, 123, 3062;
- 2bK. Shimizu, N. Imaiida, K. Kon, H. S. M. A. Siddiki, A. Satsuma, ACS Catal. 2013, 3, 998.
- 3
- 3aD. Sriram, P. Yogeeswari, R. Thirumurugan, Bioorg. Med. Chem. Lett. 2004, 14, 3923;
- 3bP. Stark, C. D. Hardison, J. Clin. Psychiatry 1985, 46, 53.
- 4
- 4aT. Yan, B. L. Feringa, K. Barta, Nat. Commun. 2014, 5, 5602;
- 4bS. Elangovan, J. Neumann, J. B. Sortais, K. Junge, C. Darce, M. Beller, Nat. Commun. 2016, 7, 12641;
- 4cG. Zhang, Z. Yin, S. Zheng, Org. Lett. 2016, 18, 300.
- 5
- 5aY. Liu, D. Cheng, H. Xu, X. Zeng, X. Wan, J. Shui, Z. Xiang, D. Cao, Proc. Natl. Acad. Sci. USA 2018, 115, 6626;
- 5bW. Liu, L. Zhang, X. Liu, X. Liu, X. Yang, S. Miao, W. Wang, A. Wang, T. Zhang, J. Am. Chem. Soc. 2017, 139, 10790;
- 5cJ. Deng, D. Deng, X. Bao, Adv. Mater. 2017, 29, 1606967;
- 5dH. Su, K. X. Zhang, B. Zhang, H. H. Wang, Q. Y. Yu, X. H. Li, M. Antonietti, J. S. Chen, J. Am. Chem. Soc. 2017, 139, 811;
- 5eS. Tian, Z. Wang, W. Gong, W. Chen, Q. Feng, Q. Xu, C. Chen, C. Chen, Q. Peng, L. Gu, H. Zhao, P. Hu, D. Wang, Y. Li, J. Am. Chem. Soc. 2018, 140, 11161.
- 6
- 6aH. B. Yang, S. F. Hung, S. Liu, K. Yuan, S. Miao, L. Zhang, X. Huang, H. Y. Wang, W. Cai, R. Chen, J. Gao, X. Yang, W. Chen, Y. Huang, H. M. Chen, C. M. Li, T. Zhang, B. Liu, Nat. Energy 2018, 3, 140;
- 6bX. Dai, Z. Chen, T. Yao, L. Zheng, Y. Lin, W. Liu, H. Ju, J. Zhu, X. Hong, S. Wei, Y. Wu, Y. Li, Chem. Commun. 2017, 53, 11568;
- 6cC. Yan, H. Li, Y. Ye, H. Wu, F. Cai, R. Si, J. Xiao, S. Miao, S. Xie, F. Yang, Y. Li, G. Wang, X. Bao, Energy Environ. Sci. 2018, 11, 1204;
- 6dC. Zhao, X. Dai, T. Yao, W. Chen, X. Wang, J. Wang, J. Yang, S. Wei, Y. Wu, Y. Li, J. Am. Chem. Soc. 2017, 139, 8078;
- 6eH. Xu, D. Cheng, D. Cao, X. C. Zeng, Nat. Catal. 2018, 1, 339;
- 6fY. Chen, S. Ji, C. Chen, Q. Peng, D. Wang, Y. Li, Joule 2018, 2, 1242.
- 7
- 7aQ. Jia, N. Ramaswamy, H. Hafiz, U. Tylus, K. Strickland, G. Wu, B. Barbiellini, A. Bansil, E. F. Holby, P. Zelenay, S. Mukerjee, ACS Nano 2015, 9, 12496;
- 7bG. J. Colpas, M. J. Maroney, C. Bagyinka, M. Kumar, W. S. Willis, S. L. Suib, N. Baidya, P. K. Mascharak, Inorg. Chem. 1991, 30, 920.
- 8
- 8aL. B. Lv, S. Yang, W. Y. Ke, H. H. Wang, B. Zhang, P. Zhang, X. H. Li, M. F. Chisholm, J. S. Chen, ChemCatChem 2018, https://doi.org/10.1002/cctc.201800707;
- 8bX. Li, W. Bi, M. Chen, Y. Sun, H. Ju, W. Yan, J. Zhu, X. Wu, W. Chu, C. Wu, Y. Xie, J. Am. Chem. Soc. 2017, 139, 14889.
- 9A. Tomer, F. Wyrwalski, C. Przybylski, J. F. Paul, E. Monflier, M. P. Titus, A. Ponchel, J. Catal. 2017, 356, 111.
- 10
- 10aF. Goettmann, A. Fischer, M. Antonietti, A. Thomas, Angew. Chem. Int. Ed. 2006, 45, 4467; Angew. Chem. 2006, 118, 4579;
- 10bH. Yang, X. Cui, X. Dai, Y. Deng, F. Shi, Nat. Commun. 2015, 6, 6478.
- 11J. S. M. Samec, J. E. Bäckvall, P. G. Andersson, P. Brandt, Chem. Soc. Rev. 2006, 35, 237.
- 12N. Zou, X. Zhou, G. Chen, N. M. Andoy, W. Jung, G. Liu, P. Chen, Nat. Chem. 2018, 10, 607.
- 13
- 13aM. J. Gilkey, B. Xu, ACS Catal. 2016, 6, 1420;
- 13bK. Yamaguchi, T. Koike, M. Kotani, M. Matsushita, S. Shinachi, N. Mizuno, Chem. Eur. J. 2005, 11, 6574;
- 13cC. M. Wong, R. T. McBurney, S. C. Binding, M. B. Peterson, V. R. Gonçales, J. J. Gooding, B. A. I. Messerle, Green Chem. 2017, 19, 3142.
- 14
- 14aA. Nilsson, L. G. M. Pettersson, B. Hammer, T. Bligaard, C. H. Christensen, J. K. Nørskov, Catal. Lett. 2005, 100, 111;
- 14bJ. K. Nørskov, F. A. Pedersena, F. Studt, T. Bligaard, Proc. Natl. Acad. Sci. USA 2011, 108, 937.