Membrane Filtration with Liquids: A Global Approach with Prior Successes, New Developments and Unresolved Challenges
Corresponding Author
Dr. Georges Belfort
Howard P. Isermann Department of Chemical and Biological Engineering and Center for Biotechnology and Interdisciplinary Studies, Rensselaer Polytechnic Institute, Troy, NY, 12180-3590 USA
Search for more papers by this authorCorresponding Author
Dr. Georges Belfort
Howard P. Isermann Department of Chemical and Biological Engineering and Center for Biotechnology and Interdisciplinary Studies, Rensselaer Polytechnic Institute, Troy, NY, 12180-3590 USA
Search for more papers by this authorGraphical Abstract
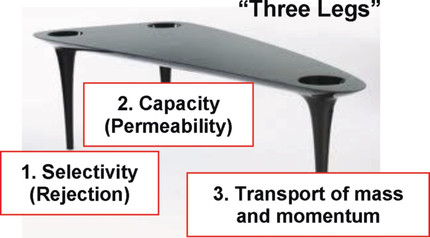
Energy-consuming thermal separation processes such as distillation will eventually be replaced by low energy-consuming processes like membrane filtration. This change has already occurred for desalination of seawater. Major challenges, however, remain with respect to increasing selectivity and permeation flux and controlling mass transport. New predictive models are needed that account for all “three legs”—selectivity, capacity and transport of mass and momentum.
Abstract
After 70 years, modern pressure-driven polymer membrane processes with liquids are mature and accepted in many industries due to their good performance, ease of scale-up, low energy consumption, modular compact construction, and low operating costs compared with thermal systems. Successful isothermal operation of synthetic membranes with liquids requires consideration of three critical aspects or “legs” in order of relevance: selectivity, capacity (i.e. permeation flow rate per unit area) and transport of mass and momentum comprising concentration polarization (CP) and fouling (F). Major challenges remain with respect to increasing selectivity and controlling mass transport in, to and away from membranes. Thus, prediction and control of membrane morphology and a deep understanding of the mechanism of dissolved and suspended solute transport near and in the membrane (i.e. diffusional and convective mass transport) is essential. Here, we focus on materials development to address the relatively poor selectivity of liquid membrane filtration with polymers and discuss the critical aspects of transport limitations. Machine learning could help optimize membrane structure design and transport conditions for improved membrane filtration performance.
Conflict of interest
The author declares no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201809548-sup-0001-misc_information.pdf9.2 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1D. S. Sholl, R. P. Lively, Nature 2016, 532, 435–437.
- 2“Market Research on Ultrafiltration Membranes: Technologies and Global Markets”: BCC Research, November 2016.
- 3S. Loeb, S. Sourirajan in Saline Water Conversion II, Vol. 38 (Ed.: ), American Chemical Society, Washington DC, 1963, pp. 117–132.
- 4
- 4aC. V. Funk, Research Scientist. Dow Water Solutions, Dow Chemcial Co., Midland MI. USA. Personal communication: “Every water is different, and every membrane is different, so experience is key. Models that exist today (like Dow's WAVE program) use extensive amounts of empirical data gathered under a wide range of conditions in combination with theory, but without the data, you have nothing—and that's just the pre-treated water. Foulants make the problem worse, and you can't predict how even a pre-treatment system will work from month to month, because water conditions change over time.” 2018;
- 4bM. Smith, Chief Technical Officer, Pall Corp., Port Washington, NY. Personal Communication: “If you look at ‘membrane based’ plant workflows—eg. Bioprocess of therapeutic protein, food an beverage production, pulp and paper, ethylene, olefin/paraffin & other refinery processes, microchip wet etching. and so on to name a few), the general opinion we have at Pall is that practitioners go through a dedicated process of scale up (small scale lab to full ‘production floor’) as an act of production readiness proof. This is done in almost all membrane-based workflow processes we see, regardless of industry. Why? Simply as you state—practitioners have to develop a prediction of workflow efficiencies, of which membrane fouling, at the variety of unit operation steps we see membranes involved, is a key contributor to energy and target product losses. Our experience tells us that for highly fouling feedstocks such as in the applications I give above—e.g. Protein in bioprocess, glucans in beer production, asphaltenes in refinery we see minimal use of membrane theory reliance but rather the drive through scale up and empirical experience.” 2018;
- 4cG. Tkacik, Director, Filtration R&D, MilliporeSigma, Bedford, MA, USA. Personal communication: “I agree that in biotech applications, the sizing of filtration steps remains highly empirical. The main reason is that predictive theoretical framework that would enable determining filtration performance from the measurable properties of the feedstream and the filter is not available at this time. Althout semi-empirical models or even microscale CFD models can be generated, because of the complexity of protein solution behavior (e.g. in situ adsorption, aggregation under dynamic filtration conditions etc.) it is not possible to robustly predict filtration performance across the range of protein feedstreams in the biotech industry.” 2018.
- 5J. Imbrogno, G. Belfort, Annu. Rev. Chem. Biomol. Eng. 2016, 7, 29–64.
- 6J. Imbrogno, J. J. Keating IV, J. E. Kilduff, G. Belfort, Desalination 2017, 401, 68–87.
- 7
- 7aH. Li, Z. Song, X. Zhang, Y. Huang, S. Li, Y. Mao, H. J. Ploehn, Y. Bao, M. Yu, Science 2013, 342, 95–98;
- 7bM. Y. Jeon, D. Kim, P. Kumar, P. S. Lee, N. Rangnekar, P. Bai, M. Shete, B. Elyassi, H. S. Lee, K. Narasimharao, S. N. Basahel, S. Al-Thabaiti, W. Xu, H. J. Cho, E. O. Fetisov, R. Thyagarajan, R. F. DeJaco, W. Fan, K. A. Mkhoyan, J. I. Siepmann, M. Tsapatsis, Nature 2017, 543, 690–694;
- 7cP. J. Bereciartua, A. Cantin, A. Corma, J. L. Jorda, M. Palomino, F. Rey, S. Valencia, E. W. Corcoran, Jr., P. Kortunov, P. I. Ravikovitch, A. Burton, C. Yoon, Y. Wang, C. Paur, J. Guzman, A. R. Bishop, G. L. Casty, Science 2017, 358, 1068–1071;
- 7dD. L. Gin, R. D. Noble, Science 2011, 332, 674–676.
- 8C. J. King, Separation Processes, 2nd ed., McGraw-Hill, New York, 1980.
- 9H. B. Park, J. Kamcev, L. M. Robeson, M. Elimelech, B. D. Freeman, Science 2017, 356, 307–311.
- 10
- 10aM. Galizia, W. S. Chi, Z. P. Smith, R. W. Baker, B. D. Freeman, Macromolecules 2017, 50, 7809–7843;
- 10bL. M. Robeson, J. Membr. Sci. 2008, 320, 390–400;
- 10cR. Swaidan, B. Ghanem, E. Litwiller, I. Pinnau, Macromolecules 2015, 48, 6553–6561.
- 11S. Y. Lee, B. S. Minhas, M. D. Donohue in New Membrane Materials and Processes for Separations, Vol. 84 (Eds.: ), AIChE, New York, 1988, p. 93.
- 12A. Mehta, A. L. Zydney, J. Membr. Sci. 2005, 249, 245–249.
- 13H. Zhang, G. M. Geise, J. Membr. Sci. 2016, 520, 790–800.
- 14A. L. Zydney, P. Aimar, M. Meireles, J. M. Pimbley, G. Belfort, J. Membr. Sci. 1994, 91, 293–298.
- 15W. J. Koros, R. Mahajan, J. Membr. Sci. 2001, 181, 141–156.
- 16
- 16aM. D. Shelby, G. L. Wilkes, Polymer 1998, 39, 6767–6779;
- 16bA. Y. Alentiev, N. A. Belov, S. V. Chirkov, Y. P. Yampolskii, J. Membr. Sci. 2018, 547, 99–109.
- 17J. D. Ferry, Chem. Rev. 1936, 18, 373–455.
- 18A. N. Cherkasov, E. A. Polotski, J. Membr. Sci. 1996, 110, 79–82.
- 19S. Dami, C. Abetz, B. Fischer, M. Radjabian, P. Georgopanos, V. Abetz, Polymer 2017, 126, 376–385.
- 20M. Mulder, Basic Principles of Membrane Technology, 2nd ed., Kluwer, Dordrecht, 1996.
10.1007/978-94-009-1766-8 Google Scholar
- 21
- 21aM. Ulbricht, O. Schuster, W. Ansorge, M. Ruetering, P. Steiger, Sep. Purif. Technol. 2007, 57, 63–73;
- 21bH. Strathmann, K. Kock, P. Amar, R. W. Baker, Desalination 1975, 16, 179–203.
- 22D. Y. Koh, B. A. McCool, H. W. Deckman, R. P. Lively, Science 2016, 353, 804–807.
- 23P. Marchetti, M. F. Jimenez Solomon, G. Szekely, A. G. Livingston, Chem. Rev. 2014, 114, 10735–10806.
- 24N. Rangnekar, N. Mittal, B. Elyassi, J. Caro, M. Tsapatsis, Chem. Soc. Rev. 2015, 44, 7128–7154.
- 25
- 25aB.-H. Jeong, E. M. V. Hoek, Y. Yan, A. Subramani, X. Huang, G. Hurwitz, A. K. Ghoask, A. Jawor, J. Membr. Sci. 2007, 294, 1–7;
- 25bM. L. Lind, D. Eumine Suk, T. V. Nguyen, E. M. Hoek, Environ. Sci. Technol. 2010, 44, 8230–8235;
- 25cM. L. Lind, A. K. Ghosh, A. Jawor, X. Huang, W. Hou, Y. Yang, E. M. Hoek, Langmuir 2009, 25, 10139–10145;
- 25dW. A. Phillip, R. M. Dorin, J. Werner, E. M. Hoek, U. Wiesner, M. Elimelech, Nano Lett. 2011, 11, 2892–2900;
- 25eS. Turgman-Cohen, J. C. Araque, E. M. Hoek, F. A. Escobedo, Langmuir 2013, 29, 12389–12399.
- 26J. E. Bachman, Z. P. Smith, T. Li, T. Xu, J. R. Long, Nat. Mater. 2016, 15, 845–849.
- 27
- 27aN. B. McKeown, P. M. Budd, Chem. Soc. Rev. 2006, 35, 675–683;
- 27bN. B. McKeown, P. M. Budd, Macromolecules 2010, 43, 5163–5176.
- 28S. P. Nunes, R. Sougrat, B. Hooghan, D. H. Anjum, A. R. Behzad, L. Zhao, N. Pradeep, I. Pinnau, U. Vainio, K.-V. Peinemann, Macromolecules 2010, 43, 8079–8085.
- 29J. M. S. Denny, J. C. Moreton, L. Benz, S. M. Cohen, Nat. Rev. Mater. 2016, 1, 1–17.
- 30R. W. Baker, Membrane Technology and Applications, 3rd ed., Wiley, Hoboken, 2012.
10.1002/9781118359686 Google Scholar
- 31M. B. Rao, S. Sirkar, J. Membr. Sci. 1993, 243, 8.
- 32J. E. Koresh, A. Soffer, Sep. Sci. Technol. 1983, 18, 723–734.
- 33K. Varoon, X. Zhang, B. Elyassi, D. D. Brewer, M. Gettel, S. Kumar, J. A. Lee, S. Maheshwari, A. Mittal, C. Y. Sung, M. Cococcioni, L. F. Francis, A. V. McCormick, K. A. Mkhoyan, M. Tsapatsis, Science 2011, 334, 72–75.
- 34X. Qiu, H. Yu, M. Karunakaran, N. Pradeep, S. P. Nunes, K. V. Peinemann, ACS Nano 2013, 7, 768–776.
- 35
- 35aC. H. Lau, P. T. Nguyen, M. R. Hill, A. W. Thornton, K. Konstas, C. M. Doherty, R. J. Mulder, L. Bourgeois, A. C. Y. Liu, D. J. Sprouster, J. P. Sullivan, T. J. Bastow, A. J. Hill, D. L. Gin, R. D. Noble, Angew. Chem. Int. Ed. 2014, 53, 5322–5326; Angew. Chem. 2014, 126, 5426–5430;
- 35bC. L. Staiger, S. J. Pas, A. J. Hill, C. J. Cornelius, Chem. Mater. 2008, 20, 2606–2608.
- 36H. Fan, J. Gu, H. Meng, A. Knebel, J. Caro, Angew. Chem. Int. Ed. 2018, 57, 4083–4087; Angew. Chem. 2018, 130, 4147–4151.
- 37M. Sára, U. Sleytr, J. Membr. Sci. 1987, 33, 27–49.
- 38C. Li, L. S. Xu, Y. Zuo, P. Yang, Biomater. Sci. 2018, 6, 836–841.
- 39J. J. Keating IV, J. Imbrogno, G. Belfort, ACS Appl. Mater. Interfaces 2016, 8, 28383–28399.
- 40
- 40aJ. J. Keating IV, M. Sorci, I. Kocsis, A. Setaro, M. Barboiu, P. Underhill, G. Belfort, J. Membr. Sci. 2017, 546, 6;
- 40bJ. J. Keating IV, A. Lee, G. Belfort, Macromolecules 2017, 50, 7930–7939.
- 41C.-C. Ho, A. L. Zydney, J. Membr. Sci. 1999, 155, 14.
- 42M. Sorci, M. Gu, C. L. Heldt, E. Grafeld, G. Belfort, Biotechnol. Bioeng. 2013, 110, 1704–1717.
- 43K. K. Yang, Z. Wu, C. N. Bedbrook, F. H. Arnold, Bioinformatics 2018, 34, 2642–2648.
- 44D. Mahlab, B. N. Joseph, G. Belfort, Chem. Eng. Commun. 1980, 6, 225–243.
- 45W. J. Koros, C. Zhang, Nat. Mater. 2017, 16, 289–297.
- 46
- 46aJ. Hermia, Trans. Inst. Chem. Engr. 1982, 60, 0;
- 46bP. Hermans, H. Bredée, J. Soc. Chem. Ind. 1963, 55, 1–4.
- 47C.-C. Ho, A. L. Zydney, J. Membr. Sci. 2000, 232, 10.
- 48J. E. Kilduff, S. Mattaraj, J. Sensibaugh, J. P. Pieracci, Y. Yuan, G. Belfort, Environ. Eng. Sci. 2002, 19, 477–495.
- 49
- 49aM. David, Y. Nissim Ben, B. Georges in Synthetic Membranes, Vol. 153, American Chemical Society, Washington, 1981, pp. 147–158;
- 49bD. Mahlab, N. Ben Yosef, G. Belfort in Synthetic Membranes (Ed.: ), American Chemical Society, Washington, 1981;
- 49cD. Mahlab, N. B. Yosef, G. Belfort, Chem. Eng. Commun. 1980, 6, 225–243.
- 50V. L. Vilker, C. K. Colton, K. A. Smith, D. L. Green, J. Membr. Sci. 1984, 20, 63–77.
- 51W. F. Blatt, A. Dravid, A. S. Michaels, L. Nelson in Membrane Science and Technology (Ed.: ), Plenum, New York, 1970.
10.1007/978-1-4684-1851-4_4 Google Scholar
- 52N. Nagata, K. J. Herouvis, D. M. Dziewulski, G. Belfort, Biotechnol. Bioeng. 1989, 34, 447–466.
- 53
- 53aG. L. Baruah, A. Venkiteshwaran, G. Belfort, Biotechnol. Prog. 2005, 21, 1013–1025;
- 53bR. H. van Eijndhoven, S. Saksena, A. L. Zydney, Biotechnol. Bioeng. 1995, 48, 406–414.
- 54
- 54aJ. Imbrogno, J. J. Keating IV, J. E. Kilduff, G. Belfort, Desalination 2016, in press;
- 54bR. W. Baker, Membrane Technology and Applications, 3rd ed., Wiley, Hoboken, 2012, chap. 9.
10.1002/9781118359686 Google Scholar
- 55O. A. N., Membrane Digest (Office of Saline Water, US Depart. of the Interior) 1972, 1, 23–57.
- 56R. W. Field, D. Wu, J. A. Howell, J. Membr. Sci. 1995, 100, 259–272.
- 57G. Belfort, R. H. Davis, A. L. Zydney, J. Membr. Sci. 1994, 96, 1–58.
- 58M. C. Porter, Ind. Eng. Chem. Prod. Res. Dev. 1972, 11, 234.
- 59J. H. Holt, H. G. Park, Y. Wang, M. Stadermann, A. B. Artyukhin, C. P. Grigoropoulos, A. Noy, O. Bakajin, Science 2006, 312, 1034–1037.
- 60
- 60aK. Sint, B. Wang, P. Kra, J. Am. Chem. Soc. 2008, 130, 16448–16449;
- 60bJ. B. Stetson, J. Mercurio, A. Rosenwinkel, P. V. Bedworth, US 20120048804A1 (Ed.: U. P. Office), Lockheed Martin Corp., USA, 2012, p. 13.
- 61R. R. Nair, H. A. Wu, P. N. Jayaram, I. V. Grigorieva, A. K. Geim, Science 2012, 335, 442–444.
- 62G. Belfort, R. J. Weigand, J. T. Mahar in ACS Symposium Series 281: Reverse Osmosis and Ultrafiltration (Eds.: ), American Chemical Society, Washington DC, 1985, pp. 383–401.
- 63W. M. Deen, Analysis of Transport Phenomena, Oxford University Press, New York, 2012.
- 64J. R. Werrber, A. Deshmukh, M. Elimelech, Environ. Sci. Technol. Lett. 2016, 3, 9.
- 65A. G. Fane, R. Wang, M. X. Hu, Angew. Chem. Int. Ed. 2015, 54, 3368–3386; Angew. Chem. 2015, 127, 3427–3447.
- 66C. Reid, E. Breton, J. Appl. Polym. Sci. 1959, 1, 133–143.
- 67
- 67aS. Loeb, S. Sourirajan, Adv. Chem. Ser. 1962, 38, 117–132;
- 67bS. Loeb, ACS Symp. Ser. 1981, 153, 9.
- 68C. Reid, Desalination by Reverse Osmosis 1966, chap. 1, pp. 1–14.
- 69
- 69aE. Glueckauf in Proc., First International Symposium on Water Desalination, Washington, DC, Vol. 1, 1965, p. 143;
- 69bG. Scatchard, J. Phys. Chem. 1964, 68, 1056–1061.
- 70S. Sourirajan, T. Matsuura, Reverse Osmnosis and Synthetic Membranes (Ed.: ), National Research Council of Canada, Ottawa, 1977.
- 71
- 71aE. Almagor, G. Belfort, J. Colloid Interface Sci. 1978, 66, 146–152;
- 71bG. Belfort, N. Sinai, Water in Polymers: 178th Meeting of the American Chemical Society, Vol. 127, American Chemical Society, Washington, 1980, chap. 19, pp. 323–345.
- 72G. Belfort, N. Sinai, Water in Polymers, ACS Symp. Series, American Chemical Society, Washington, 1980, pp. 323–346.
- 73W. A. Luck, Topics in Current Chemistry, Springer, Heidelberg, 1976, pp. 114–180.
10.1007/BFb0045699 Google Scholar
- 74
- 74aG. Belfort, Nature 1972, 237, 60–61;
- 74bG. Belfort, J. Scherfig, D. O. Seevers, J. Colloid Interface Sci. 1974, 47, 106–116.
- 75Y. Zhao, C. Qiu, X. Li, A. Vararattanavech, W. Shen, J. Torres, C. Helix-Nielsen, R. Wang, X. Hu, A. G. Fane, J. Membr. Sci. 2012, 423, 422–428.
- 76I. Kocsis, M. Sorci, H. Vanselous, S. Murail, S. E. Sanders, E. Licsandru, Y. M. Legrand, A. van der Lee, M. Baaden, P. B. Petersen, G. Belfort, M. Barboiu, Sci. Adv. 2018, 4, eaao 5603.
- 77O. R. Authored by BCS Inc. Columbia MD. and Oak Ridge National Laboratory, TN, 2005, May 4th, p. 103.