Visible-Light-Driven, Copper-Catalyzed Decarboxylative C(sp3)−H Alkylation of Glycine and Peptides
Chao Wang
Key Laboratory of Preclinical Study for New Drugs, of Gansu Province, School of Basic Medical Sciences, Lanzhou University, 199 West Donggang Road, Lanzhou, 730000 China
Search for more papers by this authorMengzhun Guo
Key Laboratory of Preclinical Study for New Drugs, of Gansu Province, School of Basic Medical Sciences, Lanzhou University, 199 West Donggang Road, Lanzhou, 730000 China
Search for more papers by this authorRupeng Qi
Key Laboratory of Preclinical Study for New Drugs, of Gansu Province, School of Basic Medical Sciences, Lanzhou University, 199 West Donggang Road, Lanzhou, 730000 China
Search for more papers by this authorQinyu Shang
Key Laboratory of Preclinical Study for New Drugs, of Gansu Province, School of Basic Medical Sciences, Lanzhou University, 199 West Donggang Road, Lanzhou, 730000 China
Search for more papers by this authorProf. Dr. Qiang Liu
State Key Laboratory of Applied Organic Chemistry, Lanzhou University, 222 South Tianshui Road, Lanzhou, 730000 China
Search for more papers by this authorShan Wang
Key Laboratory of Preclinical Study for New Drugs, of Gansu Province, School of Basic Medical Sciences, Lanzhou University, 199 West Donggang Road, Lanzhou, 730000 China
Search for more papers by this authorLong Zhao
Key Laboratory of Preclinical Study for New Drugs, of Gansu Province, School of Basic Medical Sciences, Lanzhou University, 199 West Donggang Road, Lanzhou, 730000 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Rui Wang
Key Laboratory of Preclinical Study for New Drugs, of Gansu Province, School of Basic Medical Sciences, Lanzhou University, 199 West Donggang Road, Lanzhou, 730000 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhaoqing Xu
Key Laboratory of Preclinical Study for New Drugs, of Gansu Province, School of Basic Medical Sciences, Lanzhou University, 199 West Donggang Road, Lanzhou, 730000 China
Search for more papers by this authorChao Wang
Key Laboratory of Preclinical Study for New Drugs, of Gansu Province, School of Basic Medical Sciences, Lanzhou University, 199 West Donggang Road, Lanzhou, 730000 China
Search for more papers by this authorMengzhun Guo
Key Laboratory of Preclinical Study for New Drugs, of Gansu Province, School of Basic Medical Sciences, Lanzhou University, 199 West Donggang Road, Lanzhou, 730000 China
Search for more papers by this authorRupeng Qi
Key Laboratory of Preclinical Study for New Drugs, of Gansu Province, School of Basic Medical Sciences, Lanzhou University, 199 West Donggang Road, Lanzhou, 730000 China
Search for more papers by this authorQinyu Shang
Key Laboratory of Preclinical Study for New Drugs, of Gansu Province, School of Basic Medical Sciences, Lanzhou University, 199 West Donggang Road, Lanzhou, 730000 China
Search for more papers by this authorProf. Dr. Qiang Liu
State Key Laboratory of Applied Organic Chemistry, Lanzhou University, 222 South Tianshui Road, Lanzhou, 730000 China
Search for more papers by this authorShan Wang
Key Laboratory of Preclinical Study for New Drugs, of Gansu Province, School of Basic Medical Sciences, Lanzhou University, 199 West Donggang Road, Lanzhou, 730000 China
Search for more papers by this authorLong Zhao
Key Laboratory of Preclinical Study for New Drugs, of Gansu Province, School of Basic Medical Sciences, Lanzhou University, 199 West Donggang Road, Lanzhou, 730000 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Rui Wang
Key Laboratory of Preclinical Study for New Drugs, of Gansu Province, School of Basic Medical Sciences, Lanzhou University, 199 West Donggang Road, Lanzhou, 730000 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhaoqing Xu
Key Laboratory of Preclinical Study for New Drugs, of Gansu Province, School of Basic Medical Sciences, Lanzhou University, 199 West Donggang Road, Lanzhou, 730000 China
Search for more papers by this authorGraphical Abstract
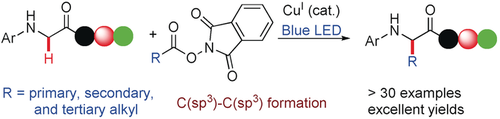
Out of the clear blue: The title reaction was developed for preparing α-alkylated non-natural α-amino acids by transformation of glycine moieties. Mild conditions and good functional-group tolerance allow the modification of peptides using this method. Mechanistic studies revealed that a radical–radical coupling pathway is involved in the reaction.
Abstract
Despite a well-developed and growing body of work in Cu catalysis, the potential of Cu to serve as a photocatalyst remains underexplored. Reported herein is the first example of visible-light-induced Cu-catalyzed decarboxylative C(sp3)−H alkylation of glycine for preparing α-alkylated unnatural α-amino acids. It merits mentioning that the mild conditions and the good functional-group tolerance allow the modification of peptides using this method. The mechanistic studies revealed that a radical–radical coupling pathway is involved in the reaction.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201809400-sup-0001-misc_information.pdf4.8 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. S. Kharasch, J. K. Hambling, T. P. Rudy, J. Org. Chem. 1959, 24, 303–305.
- 2
- 2aV. D. Parker, L. H. Piette, R. M. Salinger, C. R. Noller, J. Am. Chem. Soc. 1964, 86, 1110–1112;
- 2bV. D. Parker, C. R. Noller, J. Am. Chem. Soc. 1964, 86, 1112–1116;
- 2cM. Tamura, J. Kochi, J. Am. Chem. Soc. 1971, 93, 1483–1485;
- 2dM. Tamura, J. Kochi, J. Am. Chem. Soc. 1971, 93, 1485–1487;
- 2eM. R. Netherton, C. Dai, K. Neuschütz, G. C. Fu, J. Am. Chem. Soc. 2001, 123, 10099–10100;
- 2fT. Ishiyama, S. Abe, N. Miyaura, A. Suzuki, Chem. Lett. 1992, 21, 691–694;
- 2gA. Devasagayaraj, T. Stüdemann, P. Knochel, Angew. Chem. Int. Ed. Engl. 1996, 34, 2723–2725; Angew. Chem. 1995, 107, 2952–2954;
- 2hJ. Terao, H. Watanabe, A. Ikumi, H. Kuniyasu, N. Kambe, J. Am. Chem. Soc. 2002, 124, 4222–4223;
- 2iT. Tsuji, H. Yorimitsu, K. Oshima, Angew. Chem. Int. Ed. 2002, 41, 4137–4139;
10.1002/1521-3773(20021104)41:21<4137::AID-ANIE4137>3.0.CO;2-0 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 4311–4313;
- 2jK. B. Urkalan, M. S. Sigman, J. Am. Chem. Soc. 2009, 131, 18042;
- 2kO. Vechorkin, X. Hu, Angew. Chem. Int. Ed. 2009, 48, 2937–2940; Angew. Chem. 2009, 121, 2981–2984;
- 2lP. Ren, L.-A. Stern, X. Hu, Angew. Chem. Int. Ed. 2012, 51, 9110–9113; Angew. Chem. 2012, 124, 9244–9247;
- 2mP. M. P. Garcia, T. D. Franco, A. Orsino, P. Ren, X. Hu, Org. Lett. 2012, 14, 4286–4289.
- 3
- 3aJ. Cornella, J. T. Edwards, T. Qin, S. Kawamura, J. Wang, C.-M. Pan, R. Gianatassio, M. Schmidt, M. D. Eastgate, P. S. Baran, J. Am. Chem. Soc. 2016, 138, 2174–2177;
- 3bF. Toriyama, J. Cornella, L. Wimmer, T.-G. Chen, D. D. Dixon, G. Creech, P. S. Baran, J. Am. Chem. Soc. 2016, 138, 11132–11135;
- 3cJ. Wang, T. Qin, T.-G. Chen, L. Wimmer, J. T. Edwards, J. Cornella, B. Vokits, S. A. Shaw, P. S. Baran, Angew. Chem. Int. Ed. 2016, 55, 9676–9679; Angew. Chem. 2016, 128, 9828–9831;
- 3dJ. M. Smith, T. Qin, R. R. Merchant, J. T. Edwards, L. R. Malins, Z. Liu, G. Che, Z. Shen, S. A. Shaw, M. D. Eastgate, P. S. Baran, Angew. Chem. Int. Ed. 2017, 56, 11906–11910; Angew. Chem. 2017, 129, 12068–12072;
- 3eT. Qin, L. R. Malins, J. T. Edwards, R. R. Merchant, A. J. E. Novak, J. Z. Zhong, R. B. Mills, M. Yan, C. Yuan, M. D. Eastgate, P. S. Baran, Angew. Chem. Int. Ed. 2017, 56, 260–265; Angew. Chem. 2017, 129, 266–271;
- 3fC. Li, J. Wang, L. M. Barton, S. Yu, M. Tian, D. S. Peters, M. Kumar, A. W. Yu, K. A. Johnson, A. K. Chatterjee, M. Yan, P. S. Baran, Science 2017, 356, eaam 7355;
- 3gJ. T. Edwards, R. R. Merchant, K. S. McClymont, K. W. Knouse, T. Qin, L. R. Malins, B. Vokits, S. A. Shaw, D.-H. Bao, F.-L. We, T. Zhou, M. D. Eastgate, P. S. Baran, Nature 2017, 545, 213–218;
- 3hA. Fawcett, J. Pradeilles, Y. Wang, T. Mutsuga, E. L. Myers, V. K. Aggarwal, Science 2017, 357, 283–286;
- 3iN. Suzuki, J. L. Hofstra, K. E. Poremba, S. E. Reisman, Org. Lett. 2017, 19, 2150–2153.
- 4
- 4aT. Qin, J. Cornella, C. Li, L. R. Malins, J. T. Edwards, S. Kawamura, B. D. Maxwell, M. D. Eastgate, P. S. Baran, Science 2016, 352, 801–805;
- 4bX. Lu, B. Xiao, L. Liu, Y. Fu, Chem. Eur. J. 2016, 22, 11161–11164;
- 4cK. M. M. Huihui, J. A. Caputo, Z. Melchor, A. M. Olivares, A. M. Spiewak, K. A. Johnson, T. A. DiBenedetto, S. Kim, L. K. G. Ackerman, D. J. Weix, J. Am. Chem. Soc. 2016, 138, 5016–5019.
- 5For pioneering studies of photoinduced activation of NHP esters, see:
- 5aK. Okada, K. Okamoto, M. Oda, J. Am. Chem. Soc. 1988, 110, 8736–8738;
- 5bK. Okada, K. Okamoto, N. Morita, K. Okubo, M. Oda, J. Am. Chem. Soc. 1991, 113, 9401–9402;
- 5cM. J. Schnermann, L. E. Overman, Angew. Chem. Int. Ed. 2012, 51, 9576–9580; Angew. Chem. 2012, 124, 9714–9718;
- 5dY. Jin, H. Yang, H. Fu, Org. Lett. 2016, 18, 6400–6403;
- 5eW.-M. Cheng, R. Shang, Y. Fu, ACS Catal. 2017, 7, 907–911;
- 5fD. Hu, L. Wang, P. Li, Org. Lett. 2017, 19, 2770–2773;
- 5gY. Zhao, J.-R. Chen, W.-J. Xiao, Org. Lett. 2018, 20, 224–227;
- 5hG.-Z. Wang, R. Shang, Y. Fu, Org. Lett. 2018, 20, 888–891;
- 5iA. Tlahuext-Aca, L. Candish, R. A. Garza-Sanchez, F. Glorius, ACS Catal. 2018, 8, 1715–1719;
- 5jL. Ren, H. Cong, Org. Lett. 2018, 20, 3225–3228;
- 5kZ.-H. Xia, C.-L. Zhang, Z.-H. Gao, S. Ye, Org. Lett. 2018, 20, 3496–3499;
- 5lA. Tlahuext-Aca, R. A. Garza-Sanchez, F. Glorius, Angew. Chem. Int. Ed. 2017, 56, 3708–3711; Angew. Chem. 2017, 129, 3762–3765;
- 5mJ.-J. Zhang, J.-C. Yang, L.-N. Guo, X.-H. Duan, Chem. Eur. J. 2017, 23, 10259–10263;
- 5nW.-M. Cheng, R. Shang, M.-C. Fu, Y. Fu, Chem. Eur. J. 2017, 23, 2537–2541;
- 5oR. Mao, J. Balon, X. Hu, Angew. Chem. Int. Ed. 2018, 57, 9501–9504; Angew. Chem. 2018, 130, 9645–9648;
- 5pR. Mao, A. Frey, J. Balon, X. Hu, Nat. Catal. 2018, 1, 120–126;
- 5qR. Mao, J. Balon, X. Hu, Angew. Chem. Int. Ed. 2018, 57, 13624–13628; Angew. Chem. 2018, 130, 13812–13816.
- 6W. Zhao, R. P. Wurz, J. C. Peters, G. C. Fu, J. Am. Chem. Soc. 2017, 139, 12153–12156.
- 7C. Le, Y. Liang, R. W. Evans, X. Li, D. W. C. MacMillan, Nature 2017, 547, 79–83.
- 8W.-J. Zhou, G.-M. Cao, G. Shen, X.-Y. Zhu, Y.-Y. Gui, J.-H. Ye, L. Sun, L.-L. Liao, J. Li, D.-G. Yu, Angew. Chem. Int. Ed. 2017, 56, 15683–15687; Angew. Chem. 2017, 129, 15889–15893.
- 9For reviews, see:
- 9aS. Paria, O. Reiser, ChemCatChem 2014, 6, 2477–2483;
- 9bO. Reiser, Acc. Chem. Res. 2016, 49, 1990–1996;
- 9cA. C. Hernandez-Perez, S. K. Collins, Acc. Chem. Res. 2016, 49, 1557–1565.
- 10
- 10aE. Rémond, C. Martin, J. Martinez, F. Cavelier, Chem. Rev. 2016, 116, 11654–11684;
- 10bH. Xiao, A. Chatterjee, S.-H. Choi, K. M. Bajjuri, S. C. Sinha, P. G. Schultz, Angew. Chem. Int. Ed. 2013, 52, 14080–14083; Angew. Chem. 2013, 125, 14330–14333.
- 11
- 11aJ. Sperling, D. Elad, J. Am. Chem. Soc. 1971, 93, 967–971;
- 11bM. Schwarzberg, J. Sperling, D. Elad, J. Am. Chem. Soc. 1973, 95, 6418–6426;
- 11cH. Yu, Y. Xu, R. Dong, Y. Fang, Adv. Synth. Catal. 2017, 359, 39–43.
- 12H. Peng, J.-T. Yu, Y. Jiang, H. Yang, J. Cheng, J. Org. Chem. 2014, 79, 9847–9853.
- 13C. Wang, Y. Lei, M. Guo, Q. Shang, H. Liu, Z. Xu, R. Wang, Org. Lett. 2017, 19, 6412–6415.
- 14
- 14aY. Wang, Y. Xing, X. Liu, H. Ji, M. Kai, Z. Chen, J. Yu, D. Zhao, H. Ren, R. Wang, J. Med. Chem. 2012, 55, 6224–6236;
- 14bX. Liu, Y. Wang, Y. Xing, J. Yu, H. Ji, M. Kai, Z. Wang, D. Wang, Y. Zhang, D. Zhao, R. Wang, J. Med. Chem. 2013, 56, 3102–3114.
- 15
- 15aT. Rawner, E. Lutsker, C. A. Kaiser, O. Reiser, ACS Catal. 2018, 8, 3950–3956;
- 15bA. Hossain, A. Vidyasagar, C. Eichinger, C. Lankes, J. Phan, J. Rehbein, O. Reiser, Angew. Chem. Int. Ed. 2018, 57, 8288–8292; Angew. Chem. 2018, 130, 8420–8424.
- 16CuI(dmp)(xantphos) complex was prepared and used as photocatalyst, see:
- 16aA. C. Hernandez-Perez, S. K. Collins, Angew. Chem. Int. Ed. 2013, 52, 12696–12700; Angew. Chem. 2013, 125, 12928–12932;
- 16bP. Xiao, F. Dumur, J. Zhang, J. P. Fouassier, D. Gigmes, J. Lalevee, Macromolecules 2014, 47, 3837–3844;
- 16cC. Minozzi, A. Caron, J.-C. Grenier-Petel, J. Santandrea, S. K. Collins, Angew. Chem. Int. Ed. 2018, 57, 5477–5481; Angew. Chem. 2018, 130, 5575–5579.
- 17When Cu(MeCN)4PF6 was combined in situ with different diamine and bisphosphine ligands, and used in the standard reaction, only some of them showed catalytic activities. The HRMS analysis of the reaction mixtures indicated that the Cu(diamine)(bisphosphine)PF6 complexes were formed. In addition, the preformed Cu(diamine)(bisphosphine)PF6 complexes with various diamine and bisphosphine ligands were also applied in the standard reactions. With the same ligands, the preformed complex and the in situ generated catalyst gave the similar results. See the Supporting Information for details.