Total Syntheses of Bisdehydroneostemoninine and Bisdehydrostemoninine by Catalytic Carbonylative Spirolactonization
Dr. Kaiqing Ma
Department of Chemistry, Center for Cancer Research, and Institute for Drug Discovery, Purdue University, West Lafayette, Indiana, 47907 USA
Modern Research Center for Traditional Chinese Medicine, of Shanxi University, Taiyuan, 03006 Shanxi, China
These authors contributed equally to this work.
Search for more papers by this authorXianglin Yin
Department of Chemistry, Center for Cancer Research, and Institute for Drug Discovery, Purdue University, West Lafayette, Indiana, 47907 USA
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Mingji Dai
Department of Chemistry, Center for Cancer Research, and Institute for Drug Discovery, Purdue University, West Lafayette, Indiana, 47907 USA
Search for more papers by this authorDr. Kaiqing Ma
Department of Chemistry, Center for Cancer Research, and Institute for Drug Discovery, Purdue University, West Lafayette, Indiana, 47907 USA
Modern Research Center for Traditional Chinese Medicine, of Shanxi University, Taiyuan, 03006 Shanxi, China
These authors contributed equally to this work.
Search for more papers by this authorXianglin Yin
Department of Chemistry, Center for Cancer Research, and Institute for Drug Discovery, Purdue University, West Lafayette, Indiana, 47907 USA
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Mingji Dai
Department of Chemistry, Center for Cancer Research, and Institute for Drug Discovery, Purdue University, West Lafayette, Indiana, 47907 USA
Search for more papers by this authorGraphical Abstract
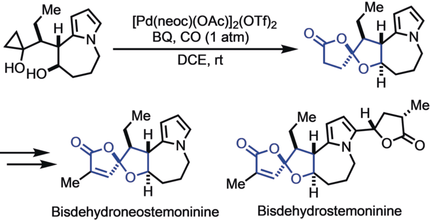
Core strength: The first total syntheses of the stemona alkaloids bisdehydroneostemoninine and bisdehydrostemoninine are reported. The synthesis features a novel palladium-catalyzed carbonylative spirolactonization to rapidly construct the oxaspirolactone moiety and a Lewis acid promoted tandem Friedel–Crafts cyclization and lactonization to form the 5-7-5 tricyclic core of the target alkaloids.
Abstract
The first total syntheses of the stemona alkaloids bisdehydroneostemoninine and bisdehydrostemoninine in racemic forms have been achieved. The synthetic strategy features a novel palladium-catalyzed carbonylative spirolactonization of a hydroxycyclopropanol to rapidly construct the oxaspirolactone moiety. It also features a Lewis acid promoted tandem Friedel–Crafts cyclization and lactonization to form the 5-7-5 tricyclic core of the target stemona alkaloids.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201809114-sup-0001-misc_information.pdf2.7 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews:
- 1aR. A. Pilli, M. C. F. de Oliveira, Nat. Prod. Rep. 2000, 17, 117–127;
- 1bR. A. Pilli, G. B. Rosso, M. C. F. de Oliveira, Nat. Prod. Rep. 2010, 27, 1908–1937;
- 1cH. Greger, Planta Med. 2006, 72, 99–113.
- 2D. C. Davis, K. L. Walker, C. Hu, R. N. Zare, R. M. Waymouth, M. Dai, J. Am. Chem. Soc. 2016, 138, 10693–10699.
- 3X. Yin, H. Mohammad, H. E. Eldesouky, A. Abdelkhalek, M. N. Seleem, M. Dai, Chem. Commun. 2017, 53, 7238–7241.
- 4L.-G. Lin, Q. X. Zhong, T. Y. Cheng, C. P. Tang, C.-Q. Ke, G. Lin, Y. Ye, J. Nat. Prod. 2006, 69, 1051–1054.
- 5P. Wang, A. L. Liu, Z. H. Li, G. H. Du, H. L. Qin, J. Asian Nat. Prod. Res. 2008, 10, 311–314.
- 6W. H. Lin, L. Ma, M. S. Cai, R. A. Barnes, Phytochemistry 1994, 36, 1333–1335.
- 7D. Kakuta, Y. Hitotsuyanagi, N. Matsuura, H. Fukaya, K. Takeya, Tetrahedron 2003, 59, 7779–7786.
- 8D. Cheng, J. Guo, T. T. Chu, J. Nat. Prod. 1988, 51, 202–211.
- 9L.-G. Lin, K. M. Li, C.-P. Tang, C.-Q. Ke, J. A. Rudd, G. Lin, Y. Ye, J. Nat. Prod. 2008, 71, 1107–1110.
- 10For reviews:
- 10aR. A. Pilli, G. B. Rosso, M. C. F. de Oliveira in The Alkaloids, Vol. 62 (Ed.: ), Elsevier, New York, 2005, pp. 77–173;
- 10bR. Alibés, M. Figueredo, Eur. J. Org. Chem. 2009, 2421–2435. For recent examples:
- 10cC. K. G. Gerlinger, S. Krüger, T. Gaich, Chem. Eur. J. 2018, 24, 3994–3997;
- 10dS. Fujita, K. Nishikawa, T. Iwata, T. Tomiyama, H. Ikenaga, K. Matsumoto, M. Shindo, Chem. Eur. J. 2018, 24, 1539–1543;
- 10eA. Varela, L. K. B. Garve, D. Leonori, V. K. Aggarwal, Angew. Chem. Int. Ed. 2017, 56, 2127–2131; Angew. Chem. 2017, 129, 2159–2163;
- 10fY. Nakayama, Y. Maeda, M. Kotatsu, R. Sekiya, M. Ichiki, T. Sato, N. Chida, Chem. Eur. J. 2016, 22, 3300–3303;
- 10gP. Q. Huang, S. Y. Huang, L. H. Gao, Z. Y. Mao, Z. Chang, A. E. Wang, Chem. Commun. 2015, 51, 4576–4578;
- 10hE. Ideue, J. Shimokawa, T. Fukuyama, Org. Lett. 2015, 17, 4964–4967;
- 10iJ. Fu, H. Shen, Y. Chang, C. Li, J. Gong, Z. Yang, Chem. Eur. J. 2015, 21, 12881–12888;
- 10jC. Fang, C. S. Shanahan, D. H. Paull, S. F. Martin, Angew. Chem. Int. Ed. 2012, 51, 10596–10599; Angew. Chem. 2012, 124, 10748–10751;
- 10kY. Wang, L. Zhu, Y. Zhang, R. Hong, Angew. Chem. Int. Ed. 2011, 50, 2787–2790; Angew. Chem. 2011, 123, 2839–2842;
- 10lZ. H. Chen, J. M. Tian, Z. M. Chen, Y. Q. Tu, Chem. Asian J. 2012, 7, 2199–2202;
- 10mZ.-H. Chen, Z.-M. Chen, Y.-Q. Zhang, Y.-Q. Tu, F.-M. Zhang, J. Org. Chem. 2011, 76, 10173–10186;
- 10nK. J. Frankowski, V. Setola, J. M. Evans, B. Neuenswander, B. L. Roth, J. Aubé, Proc. Natl. Acad. Sci. USA 2011, 108, 6727–6732;
- 10oM. Brüggemann, A. I. McDonald, L. E. Overman, M. D. Rosen, L. Schwink, J. P. Scott, J. Am. Chem. Soc. 2003, 125, 15284–15285.
- 11For a review: G. A. Brito, R. V. Pirovani, Org. Prep. Proced. Int. 2018, 50, 245–259.
- 12D. R. Williams, K. Shamim, J. P. Reddy, G. S. Amato, S. M. Shaw, Org. Lett. 2003, 5, 3361–3364.
- 13M. Yoritate, Y. Takahashi, H. Tajima, C. Ogihara, T. Yokoyama, Y. Soda, T. Oishi, T. Sato, N. Chida, J. Am. Chem. Soc. 2017, 139, 18386–18391.
- 14S. Li, F. Li, J. Gong, Z. Yang, Org. Lett. 2015, 17, 1240–1243.
- 15
- 15aC. Liu, R. A. Widenhoefer, J. Am. Chem. Soc. 2004, 126, 10250–10251;
- 15bC. Liu, R. A. Widenhoefer, Chem. Eur. J. 2006, 12, 2371–2382.
- 16B. S. Gourlay, P. P. Molesworth, J. H. Ryan, J. A. Smith, Tetrahedron Lett. 2006, 47, 799–801.
- 17B. Schmidt, S. Hauke, Org. Biomol. Chem. 2013, 11, 4194–4206.
- 18
- 18aM. G. Banwell, D. A. S. Beck, J. A. Smith, Org. Biomol. Chem. 2004, 2, 157–159;
- 18bN. A. Paras, D. W. MacMillan, J. Am. Chem. Soc. 2001, 123, 4370–4371;
- 18cR. W. Bates, S. Sridhar, J. Org. Chem. 2011, 76, 5026–5035.
- 19CCDC 1859471, 1859473, 1859474, 1859476, 1859477, 1859475, and 1859478 (7, 14, 1 a, 21, 1 b, 23 and 24) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 20
- 20aO. G. Kulinkovich, Chem. Rev. 2003, 103, 2597–2632;
- 20bI. Haym, M. A. Brimble, Org. Biomol. Chem. 2012, 10, 7649–7665.
- 21
- 21aY.-A. Zhang, Q. Liu, C. Wang, Y. Jia, Org. Lett. 2013, 15, 3662–3665.
- 21bE. Öhler, K. Reininger, U. Schmidt, Angew. Chem. Int. Ed. Engl. 1970, 9, 457; Angew. Chem. 1970, 82, 444;
- 21cA. Löffler, R. J. Pratt, H. P. Rüesch, A. S. Dreiding, Helv. Chem. Acta. 1970, 53, 383–403.
- 22
- 22aJ. S. Kingsbury, E. J. Corey, J. Am. Chem. Soc. 2005, 127, 13813–13815;
- 22bE. J. Corey, S. A. Rao, M. C. Noe, J. Am. Chem. Soc. 1994, 116, 9345–9346.
- 23E. Yoneda, S.-W. Zhang, D.-Y. Zhou, K. Onitsuka, S. Takahashi, J. Org. Chem. 2003, 68, 8571–8576.
- 24A. I. Mikhaleva, A. V. Ivanov, E. V. S. Tseva, I. A. Ushakov, A. M. V. Tsov, B. A. Trofimov, Synthesis 2009, 587–590.
- 25K. Miura, D. Wang, A. Hosomi, J. Am. Chem. Soc. 2005, 127, 9366–9367.
- 26W. Chen, Q. Yang, T. Zhou, Q. Tian, G. Zhang, Org. Lett. 2015, 17, 5236–5239.
- 27D. A. Horne, B. Fugmann, K. Yakushijin, G. Buchi, J. Org. Chem. 1993, 58, 62–64.