C2-Symmetric Bicyclic Bisborane Catalysts: Kinetic or Thermodynamic Products of a Reversible Hydroboration of Dienes
Xian-Shuang Tu
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, 94 Weijin Road, Tianjin, 300071 China
Search for more papers by this authorNing-Ning Zeng
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, 94 Weijin Road, Tianjin, 300071 China
Search for more papers by this authorRu-Ye Li
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, 94 Weijin Road, Tianjin, 300071 China
Search for more papers by this authorYu-Quan Zhao
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, 94 Weijin Road, Tianjin, 300071 China
Search for more papers by this authorDe-Zhen Xie
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, 94 Weijin Road, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Qian Peng
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, 94 Weijin Road, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiao-Chen Wang
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, 94 Weijin Road, Tianjin, 300071 China
Search for more papers by this authorXian-Shuang Tu
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, 94 Weijin Road, Tianjin, 300071 China
Search for more papers by this authorNing-Ning Zeng
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, 94 Weijin Road, Tianjin, 300071 China
Search for more papers by this authorRu-Ye Li
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, 94 Weijin Road, Tianjin, 300071 China
Search for more papers by this authorYu-Quan Zhao
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, 94 Weijin Road, Tianjin, 300071 China
Search for more papers by this authorDe-Zhen Xie
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, 94 Weijin Road, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Qian Peng
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, 94 Weijin Road, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiao-Chen Wang
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, 94 Weijin Road, Tianjin, 300071 China
Search for more papers by this authorGraphical Abstract
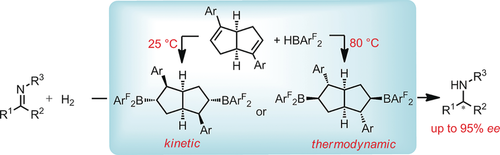
B,B bicycles: A new class of C2-symmetric bicyclic bisborane catalysts was synthesized by treating bicyclic dienes with HB(C6F5)2 or HB(p-C6F4H)2. Remarkably, two diastereomeric catalysts could be accessed selectively from a single diene precursor by simply varying the reaction temperature (see scheme). These catalysts exhibited excellent activity and selectivity in the hydrogenation of imines.
Abstract
We prepared a new class of chiral C2-symmetric bicyclic bisborane catalysts by addition reactions of internal dienes with the Piers borane, HB(C6F5)2, and an analogue, HB(p-C6F4H)2. The dependence of the addition pattern on the reaction temperature allowed us to selectively prepare two diastereomeric catalysts from a single diene precursor. The bisboranes prepared in situ exhibited excellent activity (turnover numbers up to 200 at −40 °C) and enantioselectivity (up to 95 % ee) in imine hydrogenation reactions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201808289-sup-0001-misc_information.pdf10.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1G. C. Welch, R. R. S. Juan, J. D. Masuda, D. W. Stephan, Science 2006, 314, 1124.
- 2For reviews, see:
- 2aD. W. Stephan, G. Erker, Angew. Chem. Int. Ed. 2015, 54, 6400; Angew. Chem. 2015, 127, 6498;
- 2bM. Oestreich, J. Hermeke, J. Mohr, Chem. Soc. Rev. 2015, 44, 2202;
- 2cD. W. Stephan, Science 2016, 354, aaf 7229.
- 3
- 3aD. Chen, J. Klankermayer, Chem. Commun. 2008, 2130;
- 3bD. Chen, Y. Wang, J. Klankermayer, Angew. Chem. Int. Ed. 2010, 49, 9475; Angew. Chem. 2010, 122, 9665;
- 3cD. Chen, V. Leich, F. Pan, J. Klankermayer, Chem. Eur. J. 2012, 18, 5184;
- 3dG. Ghattas, D. Chen, F. Pan, J. Klankermayer, Dalton Trans. 2012, 41, 9026.
- 4
- 4aM. Mewald, R. Fröhlich, M. Oestreich, Chem. Eur. J. 2011, 17, 9406;
- 4bM. Mewald, M. Oestreich, Chem. Eur. J. 2012, 18, 14079;
- 4cJ. Hermeke, M. Mewald, M. Oestreich, J. Am. Chem. Soc. 2013, 135, 17537;
- 4dL. Süsse, J. Hermeke, M. Oestreich, J. Am. Chem. Soc. 2016, 138, 6940.
- 5
- 5aV. Sumerin, K. Chernichenko, M. Nieger, M. Leskelä, B. Rieger, T. Repo, Adv. Synth. Catal. 2011, 353, 2093;
- 5bM. Lindqvist, K. Borre, K. Axenov, B. Kótai, M. Nieger, M. Leskelä, I. Pápai, T. Repo, J. Am. Chem. Soc. 2015, 137, 4038.
- 6
- 6aY. Liu, H. Du, J. Am. Chem. Soc. 2013, 135, 6810;
- 6bS. Wei, H. Du, J. Am. Chem. Soc. 2014, 136, 12261;
- 6cZ. Zhang, H. Du, Angew. Chem. Int. Ed. 2015, 54, 623; Angew. Chem. 2015, 127, 633;
- 6dX. Ren, H. Du, J. Am. Chem. Soc. 2016, 138, 810;
- 6eW. Meng, X. Feng, H. Du, Acc. Chem. Res. 2018, 51, 191.
- 7
- 7aX. Wang, G. Kehr, C. G. Daniliuc, G. Erker, J. Am. Chem. Soc. 2014, 136, 3293;
- 7bK.-Y. Ye, X. Wang, C. G. Daniliuc, G. Kehr, G. Erker, Eur. J. Inorg. Chem. 2017, 368.
- 8J. Lam, B. A. R. Günther, J. M. Farrell, P. Eisenberger, B. P. Bestvater, P. D. Newman, R. L. Melen, C. M. Crudden, D. W. Stephan, Dalton Trans. 2016, 45, 15303.
- 9
- 9aD. J. Parks, W. E. Piers, G. P. A. Yap, Organometallics 1998, 17, 5492;
- 9bD. J. Parks, R. E. von H. Spence, W. E. Piers, Angew. Chem. Int. Ed. Engl. 1995, 34, 809; Angew. Chem. 1995, 107, 895.
- 10For the preparation of the enantiomerically pure dienes, see:
- 10aZ.-Q. Wang, C.-G. Feng, M.-H. Xu, G.-Q. Lin, J. Am. Chem. Soc. 2007, 129, 5336;
- 10bS. Helbig, S. Sauer, N. Cramer, S. Laschat, A. Baro, W. Frey, Adv. Synth. Catal. 2007, 349, 2331;
- 10cC.-G. Feng, Z.-Q. Wang, P. Tian, M.-H. Xu, G.-Q. Lin, Chem. Asian J. 2008, 3, 1511.
- 11CCDC 1575679, 1584683, 1817472, and 1829595 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 12D. Winkelhaus, B. Neumann, H.-G. Stammler, N. W. Mitzel, Dalton Trans. 2012, 41, 8609.
- 13
- 13aP. A. Chase, T. Jurca, D. W. Stephan, Chem. Commun. 2008, 1701;
- 13bT. A. Rokob, A. Hamza, A. Stirling, I. Pápai, J. Am. Chem. Soc. 2009, 131, 2029;
- 13cT. A. Rokob, A. Hamza, I. Pápai, J. Am. Chem. Soc. 2009, 131, 10701.
- 14Since the hydroboration reactions that gave CAT5 and CAT7 also generated considerable amounts of other borane species (another bisborane isomer and a monohydroboration product, respectively), we were unable to test these two catalysts in their relatively pure forms.
- 15DFT calculations were performed using Gaussian 09, Revision D.01, M. J. Frisch, et al. Gaussian, Inc., Wallingford CT, 2009. See the Supporting Information for details.
- 16Markovnikov hydroboration and pathways giving the partially inverted product were found to be unfavorable energetically. See the Supporting Information for details.
- 17This barrier was also evaluated with another two functionals (M06-2X and B3LYP-D3), and the values were 32.2 and 28.2 kcal mol−1, respectively.
Citing Literature
November 12, 2018
Pages 15096-15100