Stack the Bowls: Tailoring the Electronic Structure of Corannulene-Integrated Crystalline Materials
Allison M. Rice
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
These authors contributed equally to this work.
Search for more papers by this authorEkaterina A. Dolgopolova
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
These authors contributed equally to this work.
Search for more papers by this authorBrandon J. Yarbrough
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
Search for more papers by this authorGabrielle A. Leith
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
Search for more papers by this authorCorey R. Martin
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
Search for more papers by this authorKenneth S. Stephenson
Department of Physics and Astronomy, USC, 712 Main Street, Columbia, SC, 29208 USA
Search for more papers by this authorRebecca A. Heugh
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
Search for more papers by this authorAmy J. Brandt
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
Search for more papers by this authorProf. Dr. Donna A. Chen
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
Search for more papers by this authorDr. Stavros G. Karakalos
College of Engineering and Computing, USC, 301 Main Street, Columbia, SC, 29208 USA
Search for more papers by this authorDr. Mark D. Smith
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
Search for more papers by this authorProf. Dr. Kelsey B. Hatzell
Department of Mechanical Engineering, Vanderbilt University, 2400 Highland Avenue, Nashville, TN, 37212 USA
Search for more papers by this authorDr. Perry J. Pellechia
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Sophya Garashchuk
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Natalia B. Shustova
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
Search for more papers by this authorAllison M. Rice
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
These authors contributed equally to this work.
Search for more papers by this authorEkaterina A. Dolgopolova
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
These authors contributed equally to this work.
Search for more papers by this authorBrandon J. Yarbrough
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
Search for more papers by this authorGabrielle A. Leith
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
Search for more papers by this authorCorey R. Martin
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
Search for more papers by this authorKenneth S. Stephenson
Department of Physics and Astronomy, USC, 712 Main Street, Columbia, SC, 29208 USA
Search for more papers by this authorRebecca A. Heugh
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
Search for more papers by this authorAmy J. Brandt
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
Search for more papers by this authorProf. Dr. Donna A. Chen
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
Search for more papers by this authorDr. Stavros G. Karakalos
College of Engineering and Computing, USC, 301 Main Street, Columbia, SC, 29208 USA
Search for more papers by this authorDr. Mark D. Smith
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
Search for more papers by this authorProf. Dr. Kelsey B. Hatzell
Department of Mechanical Engineering, Vanderbilt University, 2400 Highland Avenue, Nashville, TN, 37212 USA
Search for more papers by this authorDr. Perry J. Pellechia
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Sophya Garashchuk
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Natalia B. Shustova
Department of Chemistry and Biochemistry, University of South Carolina (USC), 631 Sumter Street, Columbia, SC, 29208 USA
Search for more papers by this authorGraphical Abstract
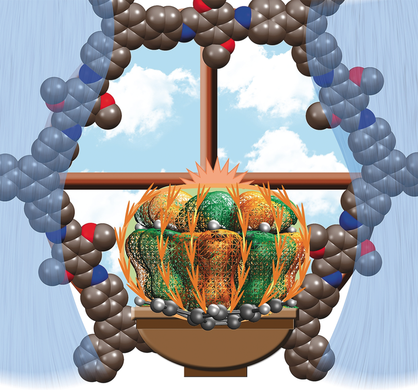
Supra-bowls: A π-bowl is ready to experience the outside world as it peers into the window of potential that could exist with its immobilization inside the scaffold. Corannulene integration resulted in the first examples of purely organic crystalline porous materials with tunable electronic profiles and four-orders-of-magnitude conductivity enhancement in comparison with the parent framework.
Abstract
We report the first examples of purely organic donor–acceptor materials with integrated π-bowls (πBs) that combine not only crystallinity and high surface areas but also exhibit tunable electronic properties, resulting in a four-orders-of-magnitude conductivity enhancement in comparison with the parent framework. In addition to the first report of alkyne–azide cycloaddition utilized for corannulene immobilization in the solid state, we also probed the charge transfer rate within the Marcus theory as a function of mutual πB orientation for the first time, as well as shed light on the density of states near the Fermi edge. These studies could foreshadow new avenues for πB utilization for the development of optoelectronic devices or a route for highly efficient porous electrodes.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201806202-sup-0001-misc_information.pdf16.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. V. Zabula, A. S. Filatov, S. N. Spisak, A. Y. Rogachev, M. A. Petrukhina, Science 2011, 333, 1008–1011.
- 2C. Dubceac, A. S. Filatov, A. V. Zabula, A. Y. Rogachev, M. A. Petrukhina, Chem. Eur. J. 2015, 21, 14268–14279.
- 3Z. Zhou, S. N. Spisak, Q. Xu, A. Y. Rogachev, Z. Wei, M. Marcaccio, M. A. Petrukhina, Chem. Eur. J. 2018, 24, 3455–3463.
- 4E. Nestoros, M. C. Stuparu, Chem. Commun. 2018, 54, 6503–6519.
- 5Y. T. Wu, J. S. Siegel, Chem. Rev. 2006, 106, 4843–4867.
- 6H. Yokoi, Y. Hiraoka, S. Hiroto, D. Sakamaki, S. Seki, H. Shinokubo, Nat. Commun. 2015, 6, 8215.
- 7A. G. Gagorik, J. W. Mohin, T. Kowalewski, G. R. Hutchison, Adv. Funct. Mater. 2015, 25, 1996–2003.
- 8L. K. San, T. T. Clikeman, C. Dubceac, A. A. Popov, Y.-S. Chen, M. A. Petrukhina, S. H. Strauss, O. V. Boltalina, Chem. Eur. J. 2015, 21, 9488–9492.
- 9M. Saito, H. Shinokubo, H. Sakurai, Mater. Chem. Front. 2018, 2, 635–661.
- 10J. Mack, P. Vogel, D. Jones, N. Kaval, A. Sutton, Org. Biomol. Chem. 2007, 5, 2448–2452.
- 11H. Wang, X. Dong, J. Lin, S. J. Teat, S. Jensen, J. Cure, E. V. Alexandrov, Q. Xia, K. Tan, Q. Wang, D. H. Olson, D. M. Proserpio, Y. J. Chabal, T. Thonhauser, J. Sun, Y. Han, J. Li, Nat. Commun. 2018, 9, 1745.
- 12Q.-G. Zhai, X. Bu, X. Zhao, D.-S. Li, P. Feng, Acc. Chem. Res. 2017, 50, 407–417.
- 13S.-T. Zheng, J. T. Bu, Y. Li, T. Wu, F. Zuo, P. Feng, X. Bu, J. Am. Chem. Soc. 2010, 132, 17062–17064.
- 14A. J. Howarth, A. W. Peters, N. A. Vermeulen, T. C. Wang, J. T. Hupp, O. K. Farha, Chem. Mater. 2017, 29, 26–39.
- 15S. M. Cohen, J. Am. Chem. Soc. 2017, 139, 2855–2863.
- 16M. K. Smith, K. A. Mirica, J. Am. Chem. Soc. 2017, 139, 16759–16767.
- 17C. R. DeBlase, W. R. Dichtel, Macromolecules 2016, 49, 5297–5305.
- 18M. Matsumoto, R. R. Dasari, W. Ji, C. H. Feriante, T. C. Parker, S. R. Marder, W. R. Dichtel, J. Am. Chem. Soc. 2017, 139, 4999–5002.
- 19C. H. Hendon, A. J. Rieth, M. D. Korzyński, M. Dincă, ACS Cent. Sci. 2017, 3, 554–563.
- 20A. Béziau, S. A. Baudron, G. Rogez, M. W. Hosseini, Inorg. Chem. 2015, 54, 2032–2039.
- 21M. W. Logan, J. D. Adamson, D. Le, F. J. Uribe-Romo, ACS Appl. Mater. Interfaces 2017, 9, 44529–44533.
- 22H. Xu, J. Gao, D. Jiang, Nat. Chem. 2015, 7, 905–912.
- 23A. M. Rice, E. A. Dolgopolova, N. B. Shustova, Chem. Mater. 2017, 29, 7054–7061.
- 24A. A. K. Karunathilake, C. M. Thompson, S. Perananthan, J. P. Ferraris, R. A. Smaldone, Chem. Commun. 2016, 52, 12881–12884.
- 25A. M. Butterfield, B. Gilomen, J. S. Siegel, Org. Process Res. Dev. 2012, 16, 664–676.
- 26N. Niamnont, N. Kimpitak, K. Wongravee, P. Rashatasakhon, K. K. Baldridge, J. S. Siegel, M. Sukwattanasinitt, Chem. Commun. 2013, 49, 780–782.
- 27A. Nagai, Z. Guo, X. Feng, S. Jin, X. Chen, X. Ding, D. Jiang, Nat. Commun. 2011, 2, 536.
- 28J. C. Bachman, R. Kavian, D. J. Graham, D. Y. Kim, S. Noda, D. G. Nocera, Y. Shao-Horn, S. W. Lee, Nat. Commun. 2015, 6, 7040.
- 29V. Rajeshkumar, M. Courte, D. Fichou, M. C. Stuparu, Eur. J. Org. Chem. 2016, 6010–6014.
- 30F. D'Souza, O. Ito, Coord. Chem. Rev. 2005, 249, 1410–1422.
- 31D. E. Williams, E. A. Dolgopolova, D. C. Godfrey, E. D. Ermolaeva, P. J. Pellechia, A. B. Greytak, M. D. Smith, S. M. Avdoshenko, A. A. Popov, N. B. Shustova, Angew. Chem. Int. Ed. 2016, 55, 9070–9074; Angew. Chem. 2016, 128, 9216–9220.
- 32E. A. Dolgopolova, A. J. Brandt, O. A. Ejegbavwo, A. S. Duke, T. D. Maddumapatabandi, R. P. Galhenage, B. W. Larson, O. G. Reid, S. C. Ammal, A. Heyden, M. Chandrashekhar, V. Stavila, D. A. Chen, N. B. Shustova, J. Am. Chem. Soc. 2017, 139, 5201–5209.
- 33R. López, R. Gómez, J. Sol-Gel Sci. Technol. 2012, 61, 1–7.
- 34M. Usman, S. Mendiratta, S. Batjargal, G. Haider, M. Hayashi, N. R. Gade, J.-W. Chen, Y.-F. Chen, K.-L. Lu, ACS Appl. Mater. Interfaces 2015, 7, 22767–22774.
- 35A. Sygula, S. Saebø, Int. J. Quantum Chem. 2009, 109, 65–72.
- 36A. S. Filatov, L. T. Scott, M. A. Petrukhina, Cryst. Growth Des. 2010, 10, 4607–4621.
- 37K. Ohta, G. L. Closs, K. Morokuma, N. J. Green, J. Am. Chem. Soc. 1986, 108, 1319–1320.
- 38A. Farazdel, M. Dupuis, E. Clementi, A. Aviram, J. Am. Chem. Soc. 1990, 112, 4206–4214.
- 39M. D. Newton, Chem. Rev. 1991, 91, 767–792.
- 40L. Y. Zhang, R. A. Friesner, R. B. Murphy, J. Chem. Phys. 1997, 107, 450–459.
- 41S. Sanyal, A. K. Manna, S. K. Pati, ChemPhysChem 2014, 15, 885–893.
- 42J. Luo, Z. Q. Xue, W. M. Liu, J. L. Wu, Z. Q. Yang, J. Phys. Chem. A 2006, 110, 12005–12009.
- 43B.-T. Wang, M. A. Petrukhina, E. R. Margine, Carbon 2015, 94, 174–180.
- 44Y.-Z. Liu, K. Yuan, Z. Yuan, Y.-C. Zhu, S.-D. Zhao, L.-L. Lv, RSC Adv. 2017, 7, 27960–27968.
- 45K. M. Pelzer, Á. Vázquez-Mayagoitia, L. E. Ratcliff, S. Tretiak, R. A. Bair, S. K. Gray, T. Van Voorhis, R. E. Larsen, S. B. Darling, Chem. Sci. 2017, 8, 2597–2609.