Catholyte-Free Electrocatalytic CO2 Reduction to Formate
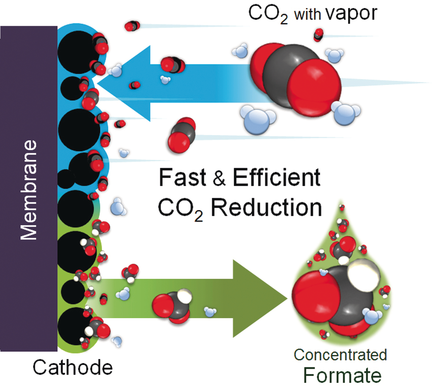
Graphical Abstract
Fast and efficient CO2 reduction: A facile strategy of catholyte-free electrocatalytic CO2 reduction (CF-CO2R) for formate production is proposed to avoid a solubility limitation in an aqueous electrolyte. The CF-CO2R method provides an increased CO2 transfer rate to the electrocatalyst and showed a significantly high formate concentration at a high current density with a high Faradaic efficiency for formate production at a low cell voltage.
Abstract
Electrochemical reduction of carbon dioxide (CO2) into value-added chemicals is a promising strategy to reduce CO2 emission and mitigate climate change. One of the most serious problems in electrocatalytic CO2 reduction (CO2R) is the low solubility of CO2 in an aqueous electrolyte, which significantly limits the cathodic reaction rate. This paper proposes a facile method of catholyte-free electrocatalytic CO2 reduction to avoid the solubility limitation using commercial tin nanoparticles as a cathode catalyst. Interestingly, as the reaction temperature rises from 303 K to 363 K, the partial current density (PCD) of formate improves more than two times with 52.9 mA cm−2, despite the decrease in CO2 solubility. Furthermore, a significantly high formate concentration of 41.5 g L−1 is obtained as a one-path product at 343 K with high PCD (51.7 mA cm−2) and high Faradaic efficiency (93.3 %) via continuous operation in a full flow cell at a low cell voltage of 2.2 V.