Highly Efficient and Stereoselective Thioallylation of Alkynes: Possible Gold Redox Catalysis with No Need for a Strong Oxidant
Dr. Jin Wang
Department of Chemistry, University of South Florida, Tampa, FL, 33620 USA
Search for more papers by this authorShuyao Zhang
Department of Chemistry, University of South Florida, Tampa, FL, 33620 USA
Search for more papers by this authorChang Xu
Department of Chemistry and Biochemistry, Ohio University, Athens, OH, 45791 USA
Search for more papers by this authorDr. Lukasz Wojtas
Department of Chemistry, University of South Florida, Tampa, FL, 33620 USA
Search for more papers by this authorDr. Novruz G. Akhmedov
C. Eugene Bennett Department of Chemistry, West Virginia University, Morgantown, WV, 26506 USA
Search for more papers by this authorProf. Dr. Hao Chen
Department of Chemistry and Biochemistry, Ohio University, Athens, OH, 45791 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaodong Shi
Department of Chemistry, University of South Florida, Tampa, FL, 33620 USA
Search for more papers by this authorDr. Jin Wang
Department of Chemistry, University of South Florida, Tampa, FL, 33620 USA
Search for more papers by this authorShuyao Zhang
Department of Chemistry, University of South Florida, Tampa, FL, 33620 USA
Search for more papers by this authorChang Xu
Department of Chemistry and Biochemistry, Ohio University, Athens, OH, 45791 USA
Search for more papers by this authorDr. Lukasz Wojtas
Department of Chemistry, University of South Florida, Tampa, FL, 33620 USA
Search for more papers by this authorDr. Novruz G. Akhmedov
C. Eugene Bennett Department of Chemistry, West Virginia University, Morgantown, WV, 26506 USA
Search for more papers by this authorProf. Dr. Hao Chen
Department of Chemistry and Biochemistry, Ohio University, Athens, OH, 45791 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaodong Shi
Department of Chemistry, University of South Florida, Tampa, FL, 33620 USA
Search for more papers by this authorGraphical Abstract
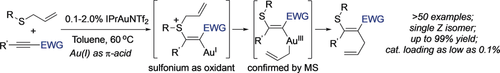
Gentle as an ox: A broad range of alkynes underwent gold-catalyzed stereoselective thioallylation with high efficiency (low catalyst loading, high yields). The gold(I) catalyst acts as both a π-acid for alkyne activation and a redox catalyst for AuI/III coupling, while the sulfonium cation generated in situ functions as a mild oxidant (see scheme).
Abstract
Stereoselective thioallylation of alkynes under possible gold redox catalysis was accomplished with high efficiency (as low as 0.1 % catalyst loading, up to 99 % yield) and broad substrate scope (various alkynes, inter- and intramolecular fashion). The gold(I) catalyst acts as both a π-acid for alkyne activation and a redox catalyst for AuI/III coupling, whereas the sulfonium cation generated in situ functions as a mild oxidant. This novel methodology provides an exciting system for gold redox catalysis without the need for a strong oxidant.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201802540-sup-0001-misc_information.pdf31.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aA. S. K. Hashmi, Gold Bull. 2004, 37, 51–65;
- 1bA. Fürstner, P. Davies, Angew. Chem. Int. Ed. 2007, 46, 3410–3449; Angew. Chem. 2007, 119, 3478–3519;
- 1cA. S. K. Hashmi, Chem. Rev. 2007, 107, 3180–3211;
- 1dE. Jiménez-Núñez, A. M. Echavarren, Chem. Rev. 2008, 108, 3326–3350;
- 1eD. J. Gorin, B. D. Sherry, F. D. Toste, Chem. Rev. 2008, 108, 3351–3378;
- 1fR. Dorel, A. M. Echavarren, Chem. Rev. 2015, 115, 9028–9072;
- 1gA. M. Asiri, A. S. K. Hashmi, Chem. Soc. Rev. 2016, 45, 4471–4503.
- 2
- 2aD. J. Gorin, F. D. Toste, Nature 2007, 446, 395–403;
- 2bM. Pernpointner, A. S. K. Hashmi, J. Chem. Theory Comput. 2009, 5, 2717–2725;
- 2cA. Leyva-Pérez, A. Corma, Angew. Chem. Int. Ed. 2012, 51, 614–635; Angew. Chem. 2012, 124, 636–658.
- 3Several vinyl gold species have been isolated; see:
- 3aJ. A. Akana, K. X. Bhattacharyya, P. Muller, J. P. Sadighi, J. Am. Chem. Soc. 2007, 129, 7736–7737;
- 3bL.-P. Liu, B. Xu, M. S. Mashuta, G. B. Hammond, J. Am. Chem. Soc. 2008, 130, 17642–17643;
- 3cD. Weber, M. A. Tarselli, M. R. Gagne, Angew. Chem. Int. Ed. 2009, 48, 5733–5736; Angew. Chem. 2009, 121, 5843–5846;
- 3dA. S. K. Hashmi, A. M. Shuster, F. Rominger, Angew. Chem. Int. Ed. 2009, 48, 8247–8249; Angew. Chem. 2009, 121, 8396–8398.
- 4
- 4aA. Buzas, F. Gagosz, Org. Lett. 2006, 8, 515–518;
- 4bM. Yu, G. Zhang, L. Zhang, Org. Lett. 2007, 9, 2147–2150;
- 4cD. Wang, X. Ye, X. Shi, Org. Lett. 2010, 12, 2088–2091;
- 4dT. Wang, S. Shi, M. Rudolph, A. S. K. Hashmi, Adv. Synth. Catal. 2014, 356, 2337–2342.
- 5H. Peng, N. G. Akhmedov, Y. Liang, N. Jiao, X. Shi, J. Am. Chem. Soc. 2015, 137, 8912–8915.
- 6For gold/metal dual catalysis, see:
- 6aJ. Hirner, Y. Shi, S. Blum, Acc. Chem. Res. 2011, 44, 603–613;
- 6bA. S. K. Hashmi, C. Lothschütz, R. Dopp, M. Ackermann, J. D. B. Becker, M. Rudolph, C. Scholz, F. Rominger, Adv. Synth. Catal. 2012, 354, 133–147;
- 6cP. García-Domínguez, C. Nevado, J. Am. Chem. Soc. 2016, 138, 3266–3269.
- 7For examples of the oxidative addition of AuI to alkyl halides, see:
- 7aA. Tamaki, J. K. Kochi, J. Organomet. Chem. 1972, 40, C 81–C84;
- 7bA. Tamaki, J. K. Kochi, J. Chem. Soc. Dalton Trans. 1973, 23, 2620–2626;
- 7cA. Tamaki, J. K. Kochi, Inorg. Nucl. Chem. Lett. 1973, 9, 1175–1177;
- 7dA. Tamaki, J. K. Kochi, J. Organomet. Chem. 1974, 64, 411–425;
- 7eA. Johnson, R. J. Puddephatt, J. Organomet. Chem. 1975, 85, 115–121;
- 7fM. D. Levin, F. D. Toste, Angew. Chem. Int. Ed. 2014, 53, 6211–6215; Angew. Chem. 2014, 126, 6325–6329;
- 7gM. S. Winston, W. J. Wolf, F. D. Toste, J. Am. Chem. Soc. 2014, 136, 7777–7782.
- 8For reviews on AuI/III oxidative coupling, see:
- 8aM. N. Hopkinson, A. D. Gee, V. Gouverneur, Chem. Eur. J. 2011, 17, 8248–8262;
- 8bH. A. Wegner, M. Auzias, Angew. Chem. Int. Ed. 2011, 50, 8236–8247; Angew. Chem. 2011, 123, 8386–8397; for pioneer studies on AuI/III cross-coupling, see:
- 8cG. Zhang, Y. Peng, L. Cui, L. Zhang, Angew. Chem. Int. Ed. 2009, 48, 3112–3115; Angew. Chem. 2009, 121, 3158–3161;
- 8dA. S. K. Hashmi, T. D. Ramamurthi, F. Rominger, J. Organomet. Chem. 2009, 694, 592–597;
- 8eM. Pažický, A. Loos, M. J. Ferreira, D. Serra, N. Vinokunov, F. Rominger, C. Jäkel, A. S. K. Hashmi, M. Limbach, Organometallics 2010, 29, 4448–4458.
- 9For early examples of gold(III)-mediated nucleophilic addition–homocoupling sequences, see:
- 9aA. S. K. Hashmi, M. C. Blanco, D. Fisher, J. W. Bats, Eur. J. Org. Chem. 2006, 1387–1389;
- 9bH. A. Wegner, S. Ahles, M. Neuburger, Chem. Eur. J. 2008, 14, 11310–11313;
- 9cS. Fustero, P. Bello, B. Fernandez, C. del Pozo, G. B. Hammond, J. Org. Chem. 2009, 74, 7690–7696; for gold(I)-catalyzed nucleophilic addition–cross-coupling sequences, see:
- 9dG. Zhang, L. Cui, Y. Wang, L. Zhang, J. Am. Chem. Soc. 2010, 132, 1474–1475;
- 9eA. D. Melhado, W. E. Brenzovich, Jr., A. D. Lacktner, F. D. Toste, J. Am. Chem. Soc. 2010, 132, 8885–8887;
- 9fW. E. Brenzovich, Jr., D. Benitez, A. D. Lacktner, H. P. Shunatona, E. Tkatchouk, W. A. Goddard III, F. D. Toste, Angew. Chem. Int. Ed. 2010, 49, 5519–5522; Angew. Chem. 2010, 122, 5651–5654.
- 10For photoredox conditions with gold catalysts and photoactive dyes, see:
- 10aB. Sahoo, M. N. Hopkinson, F. Glorius, J. Am. Chem. Soc. 2013, 135, 5505–5508;
- 10bX. Shu, M. Zhang, Y. He, H. Frei, F. D. Toste, J. Am. Chem. Soc. 2014, 136, 5844–5847;
- 10cM. N. Hopkinson, A. Tlahuext-Aca, F. Glorius, Acc. Chem. Res. 2016, 49, 2261–2272; for photoredox conditions with photoactive gold catalysts only and no additional dyes, see:
- 10dJ. Xie, S. Shi, T. Zhang, N. Mehrkens, M. Rudolph, A. S. K. Hashmi, Angew. Chem. Int. Ed. 2015, 54, 6046–6050; Angew. Chem. 2015, 127, 6144–6148;
- 10eJ. Xie, T. Zhang, F. Chen, N. Mehrkens, F. Rominger, M. Rudolph, A. S. K. Hashmi, Angew. Chem. Int. Ed. 2016, 55, 2934–2938; Angew. Chem. 2016, 128, 2987–2991;
- 10fL. Huang, M. Rudolph, F. Rominger, A. S. K. Hashmi, Angew. Chem. Int. Ed. 2016, 55, 4808–4813; Angew. Chem. 2016, 128, 4888–4893;
- 10gJ. Xie, J. Li, V. Weingand, M. Rudolph, A. S. K. Hashmi, Chem. Eur. J. 2016, 22, 12646–12650;
- 10hL. Huang, F. Rominger, M. Rudolph, A. S. K. Hashmi, Chem. Commun. 2016, 52, 6435–6438;
- 10iS. Witzel, J. Xie, M. Rudolph, A. S. K. Hashmi, Adv. Synth. Catal. 2017, 359, 1522–1528; for basic conditions, see:
- 10jR. Cai, M. Lu, E. Y. Aguilera, Y. Xi, N. G. Akmedov, J. L. Petersen, H. Chen, X. Shi, Angew. Chem. Int. Ed. 2015, 54, 8772–8776; Angew. Chem. 2015, 127, 8896–8900;
- 10kB. Dong, H. Peng, S. E. Motika, X. Shi, Chem. Eur. J. 2017, 23, 11093–11099.
- 11For Meyer–Schuster-type rearrangement, see:
- 11aRef. [8c];
- 11bY. Yu, W. Yang, D. Pflasterer, A. S. K. Hashmi, Angew. Chem. Int. Ed. 2014, 53, 1144–1147; Angew. Chem. 2014, 126, 1162–1165;
- 11cJ. Um, H. Yun, S. Shin, Org. Lett. 2016, 18, 484–487;
- 11dA. Tlahuext-Aca, M. N. Hopkinson, R. A. Garza-Sanchez, F. Glorius, Chem. Eur. J. 2016, 22, 5909–5913; for a Sonogashira-type coupling reaction, see:
- 11eA. Tlahuext-Aca, M. N. Hopkinson, B. Sahoo, F. Glorius, Chem. Sci. 2016, 7, 89–93; for oxyarylation and aminoarylation, see:
- 11fRef. [10f];
- 11gZ. Wang, T. Tan, C. Wang, D. Yuan, T. Zhang, P. Zhu, C. Zhu, J. Zhou, L. Ye, Chem. Commun. 2017, 53, 6848–6851.
- 12X. Ye, J. Wang, S. Ding, S. Hosseyni, L. Wojtas, N. G. Akhmedov, X. Shi, Chem. Eur. J. 2017, 23, 10506–10510.
- 13The Hashmi group reported an intramolecular oxyallylation reaction in an analogous allyl 2-ethynylphenyl ether system; a mechanism involving [3,3] sigmatropic rearrangement was proposed, which was supported by a cross-over experiment and a computational study:
- 13aA. S. K. Hashmi, K. Graf, M. Ackermann, F. Rominger, ChemCatChem 2013, 5, 1200–1204;
- 13bM. Ackermann, J. Bucher, M. Rappold, K. Graf, F. Rominger, A. S. K. Hashmi, Chem. Asian J. 2013, 8, 1786–1794;
- 13cL. Nunes dos Santos Comprido, J. E. M. N. Klein, G. Knizia, J. Kastner, A. S. K. Hashmi, Chem. Eur. J. 2017, 23, 10901–10905.
- 14K. Hayakawa, Y. Kamikawaji, K. Kanematsu, Tetrahedron Lett. 1982, 23, 2171–2174.
- 15
- 15aK. A. Dekorver, H. Li, A. G. Lohse, R. Hayashi, Z. Lu, Y. Zhang, R. P. Hsung, Chem. Rev. 2010, 110, 5064–5106;
- 15bG. Evano, A. Coste, K. Jouvin, Angew. Chem. Int. Ed. 2010, 49, 2840–2859; Angew. Chem. 2010, 122, 2902–2921.
- 16K. S. Sindhu, A. P. Thankachan, P. S. Sajitha, G. Anikumar, Org. Biomol. Chem. 2015, 13, 6891–6905.
- 17W. Wu, H. Jiang, Acc. Chem. Res. 2014, 47, 2483–2504.