Hydrophilic Oligo(lactic acid)s Captured by a Hydrophobic Polyaromatic Cavity in Water
Shunsuke Kusaba
Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 Japan
Search for more papers by this authorDr. Masahiro Yamashina
Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 Japan
Search for more papers by this authorProf. Dr. Munetaka Akita
Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 Japan
Search for more papers by this authorDr. Takashi Kikuchi
Rigaku Corporation, 3-9-12 Matsubaracho, Akishima Tokyo 196–8666, Japan
Search for more papers by this authorCorresponding Author
Dr. Michito Yoshizawa
Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 Japan
Search for more papers by this authorShunsuke Kusaba
Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 Japan
Search for more papers by this authorDr. Masahiro Yamashina
Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 Japan
Search for more papers by this authorProf. Dr. Munetaka Akita
Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 Japan
Search for more papers by this authorDr. Takashi Kikuchi
Rigaku Corporation, 3-9-12 Matsubaracho, Akishima Tokyo 196–8666, Japan
Search for more papers by this authorCorresponding Author
Dr. Michito Yoshizawa
Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 Japan
Search for more papers by this authorGraphical Abstract
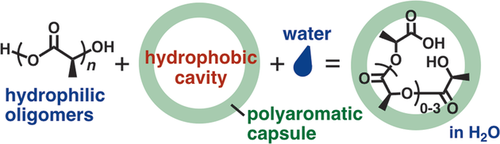
Incompatible interactions: The hydrophobic cavity of a polyaromatic capsule efficiently encapsulated hydrophilic oligo(lactic acid)s in water (see scheme). The X-ray crystallographic and ITC analyses revealed that the unusual host–guest behavior is caused by enthalpic stabilization through multiple CH–π and hydrogen bonding interactions.
Abstract
Biologically relevant hydrophilic molecules rarely interact with hydrophobic compounds and surfaces in water owing to effective hydration. Nevertheless, herein we report that the hydrophobic cavity of a polyaromatic capsule, formed through coordination-driven self-assembly, can encapsulate hydrophilic oligo(lactic acid)s in water with relatively high binding constants (up to Ka=3×105 m−1). X-ray crystallographic and ITC analyses revealed that the unusual host–guest behavior is caused by enthalpic stabilization through multiple CH–π and hydrogen-bonding interactions. The polyaromatic cavity stabilizes hydrolyzable cyclic di(lactic acid) and captures tetra(lactic acid) preferentially from a mixture of oligo(lactic acid)s even in water.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201800432-sup-0001-misc_information.pdf4.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. W. Steed, J. L. Atwood, Supramolecular Chemistry, 2nd ed., Wiley, Hoboken, 2009.
10.1002/9780470740880 Google Scholar
- 2For recent reviews, see:
- 2aM. D. Ward, P. R. Raithby, Chem. Soc. Rev. 2013, 42, 1619–1636;
- 2bM. M. J. Smulders, I. A. Riddell, C. Browne, J. R. Nitschke, Chem. Soc. Rev. 2013, 42, 1728–1754;
- 2cK. Harris, D. Fujita, M. Fujita, Chem. Commun. 2013, 49, 6703–6712;
- 2dM. Han, D. M. Engelhard, G. H. Clever, Chem. Soc. Rev. 2014, 43, 1848–1860;
- 2eL. Xu, L.-J. Chen, H.-B. Yang, Chem. Commun. 2014, 50, 5156–5170;
- 2fA. M. Castilla, W. J. Ramsay, J. R. Nitschke, Acc. Chem. Res. 2014, 47, 2063–2073;
- 2gT. R. Cook, P. J. Stang, Chem. Rev. 2015, 115, 7001–7045;
- 2hC. J. Brown, F. D. Toste, R. G. Bergman, K. N. Raymond, Chem. Rev. 2015, 115, 3012–3035;
- 2iM. Yoshizawa, M. Yamashina, Chem. Lett. 2017, 46, 163–171;
- 2jR. A. S. Vasdev, D. Preston, J. D. Crowley, Chem. Asian J. 2017, 12, 2513–2523;
- 2kL.-J. Chen, H.-B. Yang, M. Shionoya, Chem. Soc. Rev. 2017, 46, 2555–2576.
- 3For examples of molecular cages for hydrophilic (polar) substrates in water, see:
- 3aS. Tashiro, M. Tominaga, M. Kawano, B. Therrien, T. Ozeki, M. Fujita, J. Am. Chem. Soc. 2005, 127, 4546–4547;
- 3bS. Tashiro, M. Kobayashi, M. Fujita, J. Am. Chem. Soc. 2006, 128, 9280–9281;
- 3cT. Sawada, M. Yoshizawa, S. Sato, M. Fujita, Nat. Chem. 2009, 1, 53–56;
- 3dB. Sookcharoenpinyo, E. Klein, Y. Ferrand, D. B. Walker, P. R. Brotherhood, C. Ke, M. P. Crump, A. P. Davis, Angew. Chem. Int. Ed. 2012, 51, 4586–4590; Angew. Chem. 2012, 124, 4664–4668;
- 3eP. Rios, T. S. Carter, T. J. Mooibroek, M. P. Crump, M. Lisbjerg, M. Pittelkow, N. T. Supekar, G.-J. Boons, A. P. Davis, Angew. Chem. Int. Ed. 2016, 55, 3387–3392; Angew. Chem. 2016, 128, 3448–3453.
- 4N. Kishi, Z. Li, K. Yoza, M. Akita, M. Yoshizawa, J. Am. Chem. Soc. 2011, 133, 11438–11441.
- 5M. Yamashina, M. Akita, T. Hasegawa, S. Hayashi, M. Yoshizawa, Sci. Adv. 2017, 3, e 1701126.
- 6A. Galana, P. Ballester, Chem. Soc. Rev. 2016, 45, 1720–1737.
- 7
- 7a Polylactic Acid Science and Technology: Processing, Properties, Additives, and Applications (Eds.: ), RSC, London, 2015;
- 7b Synthesis, Structure and Properties of Poly(lactic acid) (Eds.: ), Springer, Berlin, 2018.
- 8
- 8aS. Inkinen, M. Hakkarainen, A.-C. Albertsson, A. Södergård, Biomacromolecules 2011, 12, 523–532;
- 8bS. Lazzari, F. Codari, G. Storti, M. Morbidelli, D. Moscatelli, Polym. Degrad. Stab. 2014, 110, 80–90;
- 8cN. Kameno, S. Yamada, T. Amimoto, K. Amimoto, H. Ikeda, N. Koga, Polym. Degrad. Stab. 2016, 134, 284–295;
- 8dK. Nomura, H. Ohara, Mini-Rev. Org. Chem. 2017, 14, 35–43.
- 9
- 9aFor a synthetic host for lactic acid monomer (up to Ka≈700 m−1) in CHCl3, see: T. Barboza, R. Pinalli, C. Massera, E. Dalcanale, CrystEngComm 2016, 18, 4958–4963;
- 9bfor biological hosts (i.e., lactate dehydrogenases) for lactic acid monomer, see: J. J. Holbrook, A. Liljas, S. J. Steindel, M. G. Rossmann, The Enzymes 1975, 11, 191–292.
- 10For host capability toward hydrophobic molecules, see:
- 10aN. Kishi, Z. Li, Y. Sei, M. Akita, K. Yoza, J. S. Siegel, M. Yoshizawa, Chem. Eur. J. 2013, 19, 6313–6320;
- 10bN. Kishi, M. Akita, M. Yoshizawa, Angew. Chem. Int. Ed. 2014, 53, 3604–3607; Angew. Chem. 2014, 126, 3678–3681;
- 10cM. Yamashina, Y. Sei, M. Akita, M. Yoshizawa, Nat. Commun. 2014, 5, 4662;
- 10dM. Yamashina, M. Sartin, Y. Sei, M. Akita, S. Takeuchi, T. Tahara, M. Yoshizawa, J. Am. Chem. Soc. 2015, 137, 9266–9269;
- 10eM. Yamashina, S. Matsuno, Y. Sei, M. Akita, M. Yoshizawa, Chem. Eur. J. 2016, 22, 14147–14150;
- 10fS. Matsuno, M. Yamashina, Y. Sei, M. Akita, A. Kuzume, K. Yamamoto, M. Yoshizawa, Nat. Commun. 2017, 8, 749.
- 11See the Supporting Information.
- 12The 1H NMR spectrum of 1 in D2O showed desymmetrized signals derived from the anthracene moieties: the protons Hb–e split into eight signals (Figure 2 b). The proton signals of 2 b were also desymmetrized in the confined cavity (Figure 2 c). 1H DOSY measurement revealed that the host (Ha-l) and guest (HA–D) signals are on a single band (see Figure S4 in the Supporting Information).[11]
- 13Pale-yellow single crystals were obtained by slow concentration of a solution of 1⋅(2 b)2 and 1⋅4 b in H2O at room temperature for 45 and 30 days, respectively.[11]
- 14
- 14aK. Yazaki, N. Kishi, M. Akita, M. Yoshizawa, Chem. Commun. 2013, 49, 1630–1632;
- 14bD. P. August, G. S. Nichol, P. J. Lusby, Angew. Chem. Int. Ed. 2016, 55, 15022–15026; Angew. Chem. 2016, 128, 15246–15250.
- 15In the 1H NMR spectrum of 1⋅(2 b)2 (Figure 2 c), the downfield shift (Δδ=+0.03 ppm) of the pyridine α-hydrogen atom (Hf) of 1 is also indicative of the host–guest hydrogen-bonding interactions in water.
- 16The ESI-TOF MS spectrum of 1⋅(3 b)2 showed prominent peaks at m/z 1925.9, 1263.3, and 932.0, assignable to the [1⋅(3 b)2–n⋅NO3−]n+ (n=2–4) species (see Figure S18).[11]
- 17The minimum value of the capsule cavity was calculated from the crystal structure of 1⋅(1-adamantanecarboxylic acid) by using Material Studio (Accelrys Software Inc., Connolly radius=1.4 Å).[10a]
- 18C. F. van Nostrum, T. F. J. Veldhuis, G. W. Bos, W. E. Hennink, Polymer 2004, 45, 6779–6787.
- 19FT-IR analysis of 1⋅4 b, 1⋅(3 b)2, and 1⋅(2 b)2 also indicated the presence of host–guest hydrogen-bonding interactions (see Figure S40).[11]
- 20Tetramer 4 b is well soluble in water (>20 mm) and cannot be extracted with CHCl3 and AcOEt from the aqueous solution, probably because the carbonyl, carboxy, and hydroxy groups on the helical framework (Figure 4 d) are involved in efficient hydrogen-bonding interactions with solvating water molecules. The dipole moments of 4 b and dimer 3 a, estimated preliminarily by DFT calculations (B3LYP/6-31G* level, gas phase), are relatively high (4.7 and 3.7 D, respectively).
- 21
- 21aF. Biedermann, V. D. Uzunova, O. A. Scherman, W. M. Nau, A. De Simone, J. Am. Chem. Soc. 2012, 134, 15318–15323;
- 21bF. Biedermann, W. M. Nau, H.-J. Schneider, Angew. Chem. Int. Ed. 2014, 53, 11158–11171; Angew. Chem. 2014, 126, 11338–11352;
- 21cA. J. Metherell, W. Cullen, N. H. Williams, M. D. Ward, Chem. Eur. J. 2018, 24, 1554-1560.
- 22The preliminary X-ray crystallographic analysis of empty capsule 1 suggested the presence of water molecules, most of which are severely disordered, in the cavity. The cavity volume of 1 is changeable (ca. 430–690 Å3) depending on the size, shape, and number of the encapsulated guest.[10a] Thus, further studies are needed to discuss the hydrophobic effect in the present host-guest system.