Direct Cyclization of Tertiary Aryl Amines with Iodonium Ylides
Zhiguo Zhao
School of Chemistry and Chemical Engineering, Key Laboratory of the Colloid and Interface Chemistry, Ministry of Education, Shandong University, 27 Shanda Nanlu, Jinan, 250100 Shandong, China
Search for more papers by this authorYongrui Luo
School of Chemistry and Chemical Engineering, Key Laboratory of the Colloid and Interface Chemistry, Ministry of Education, Shandong University, 27 Shanda Nanlu, Jinan, 250100 Shandong, China
Search for more papers by this authorDr. Shuya Liu
School of Chemistry and Chemical Engineering, Key Laboratory of the Colloid and Interface Chemistry, Ministry of Education, Shandong University, 27 Shanda Nanlu, Jinan, 250100 Shandong, China
Search for more papers by this authorLiang Zhang
School of Chemistry and Chemical Engineering, Key Laboratory of the Colloid and Interface Chemistry, Ministry of Education, Shandong University, 27 Shanda Nanlu, Jinan, 250100 Shandong, China
Search for more papers by this authorDr. Lei Feng
School of Chemistry and Chemical Engineering, Key Laboratory of the Colloid and Interface Chemistry, Ministry of Education, Shandong University, 27 Shanda Nanlu, Jinan, 250100 Shandong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yao Wang
School of Chemistry and Chemical Engineering, Key Laboratory of the Colloid and Interface Chemistry, Ministry of Education, Shandong University, 27 Shanda Nanlu, Jinan, 250100 Shandong, China
Search for more papers by this authorZhiguo Zhao
School of Chemistry and Chemical Engineering, Key Laboratory of the Colloid and Interface Chemistry, Ministry of Education, Shandong University, 27 Shanda Nanlu, Jinan, 250100 Shandong, China
Search for more papers by this authorYongrui Luo
School of Chemistry and Chemical Engineering, Key Laboratory of the Colloid and Interface Chemistry, Ministry of Education, Shandong University, 27 Shanda Nanlu, Jinan, 250100 Shandong, China
Search for more papers by this authorDr. Shuya Liu
School of Chemistry and Chemical Engineering, Key Laboratory of the Colloid and Interface Chemistry, Ministry of Education, Shandong University, 27 Shanda Nanlu, Jinan, 250100 Shandong, China
Search for more papers by this authorLiang Zhang
School of Chemistry and Chemical Engineering, Key Laboratory of the Colloid and Interface Chemistry, Ministry of Education, Shandong University, 27 Shanda Nanlu, Jinan, 250100 Shandong, China
Search for more papers by this authorDr. Lei Feng
School of Chemistry and Chemical Engineering, Key Laboratory of the Colloid and Interface Chemistry, Ministry of Education, Shandong University, 27 Shanda Nanlu, Jinan, 250100 Shandong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yao Wang
School of Chemistry and Chemical Engineering, Key Laboratory of the Colloid and Interface Chemistry, Ministry of Education, Shandong University, 27 Shanda Nanlu, Jinan, 250100 Shandong, China
Search for more papers by this authorGraphical Abstract
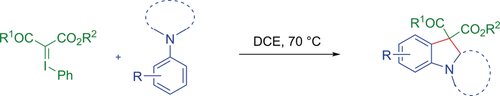
In an impressive show of independence, iodonium ylides underwent direct cyclization with unmodified tertiary aryl amines (see scheme). The transformation, which was developed on the basis of the exceptional finding that iodonium ylides could cleave the C(sp3)−H bonds of tertiary aryl amines, proceeded in the absence of a transition-metal catalyst and without the use of an additional initiator/oxidant.
Abstract
Described herein is a direct cyclization of simple tertiary aryl amines with iodonium ylides leading to a broad range of N-heterocycles. Completely different from the known reactivity of iodonium ylides, the finding reported herein is that an iodonium ylide is capable of cleaving a C(sp3)−H bond and accepting two hydrogen atoms of a tertiary aryl amine, thus inducing a novel cyclization process. This transformation can proceed without the assistance of a transition-metal catalyst and eliminates the need for the premodification of the amine or the use of an additional initiator/oxidant.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201800389-sup-0001-misc_information.pdf2.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1E. Gudrinietse, O. Neiland, G. Vanags, Zh. Obshch. Khim. 1957, 27, 2737–2740.
- 2
- 2aP. J. Stang, V. V. Zhdankin, Chem. Rev. 1996, 96, 1123–1178;
- 2bA. Varvoglis, Hypervalent Iodine in Organic Synthesis, Academic Press, London, 1997;
10.1016/B978-012714975-2/50011-9 Google Scholar
- 2cV. V. Zhdankin, P. J. Stang, Chem. Rev. 2002, 102, 2523–2584;
- 2dA. Yoshimura, V. V. Zhdankin, Chem. Rev. 2016, 116, 3328–3435.
- 3
- 3aP. Müller, Acc. Chem. Res. 2004, 37, 243–251;
- 3bV. V. Zhdankin, Hypervalent Iodine Chemistry: Preparation, Structure and Synthetic Applications of Polyvalent Iodine Compounds, Wiley, New York, 2013.
10.1002/9781118341155 Google Scholar
- 4For selected examples, see:
- 4aJ. Hackenberg, M. Hanack, J. Chem. Soc. Chem. Commun. 1991, 470–471;
- 4bR.-Y. Yang, L.-X. Dai, C.-C. Chen, J. Chem. Soc. Chem. Commun. 1992, 1487–1488;
- 4cM. Ochiai, T. Okada, N. Tada, A. Yoshimura, Org. Lett. 2008, 10, 1425–1428;
- 4dJ. Vaitla, K. H. Hopmann, A. Bayer, Org. Lett. 2017, 19, 6688–6691.
- 5For selected examples, see:
- 5aS. R. Goudreau, D. Marcoux, A. B. Charette, J. Org. Chem. 2009, 74, 470–473;
- 5bC. Zhu, A. Yoshimura, L. Ji, Y. Wei, V. N. Nemykin, V. V. Zhdankin, Org. Lett. 2012, 14, 3170–3173;
- 5cC. Deng, L.-J. Wang, J. Zhu, Y. Tang, Angew. Chem. Int. Ed. 2012, 51, 11620–11623; Angew. Chem. 2012, 124, 11788–11791;
- 5dJ. Guo, Y. Liu, X. Li, X. Liu, L. Lin, X. Feng, Chem. Sci. 2016, 7, 2717–2721.
- 6For a review, see:
- 6aM. Jia, S. Ma, Angew. Chem. Int. Ed. 2016, 55, 9134–9166; Angew. Chem. 2016, 128, 9280–9313; for selected examples, see:
- 6bM. B. Camacho, A. E. Clark, T. A. Liebrecht, J. P. Deluca, J. Am. Chem. Soc. 2000, 122, 5210–5211;
- 6cP. Müller, C. Bolea, Helv. Chim. Acta 2002, 85, 483–494;
- 6dC. Batsila, E. P. Gogonas, G. Kostakis, L. P. Hadjiarapoglou, Org. Lett. 2003, 5, 1511–1514;
- 6eS. Telu, S. Durmus, G. F. Koser, Tetrahedron Lett. 2007, 48, 1863–1866.
- 7
- 7aO. I. Kolodiazhnyi, Phosphorus Ylides: Chemistry and Applications in Organic Chemistry, Wiley-VCH, Weinheim, 1999;
10.1002/9783527613908 Google Scholar
- 7bA.-H. Li, L.-X. Dai, V. K. Aggarwal, Chem. Rev. 1997, 97, 2341–2372.
- 8
- 8aM. Ochiai, Y. Kitagawa, S. Yamamoto, J. Am. Chem. Soc. 1997, 119, 11598–11604;
- 8bM. Ochiai, Y. Kitagawa, J. Org. Chem. 1999, 64, 3181–3189;
- 8cX.-C. Huang, Y.-L. Liu, Y. Liang, S.-F. Pi, F. Wang, J.-H. Li, Org. Lett. 2008, 10, 1525–1528;
- 8dA. Antos, Y. Elemes, A. Michaelides, J. A. Nyxas, S. Skoulika, L. P. Hadjiarapoglou, J. Org. Chem. 2012, 77, 10949–10954;
- 8eH. Huang, Y. Yang, X. Zhang, W. Zeng, Y. Liang, Tetrahedron Lett. 2013, 54, 6049–6052;
- 8fS. Chelli, K. Troshin, P. Mayer, S. Lakhdar, A. R. Ofial, H. Mayr, J. Am. Chem. Soc. 2016, 138, 10304–10313.
- 9
- 9aV. V. Zhdankin, P. J. Stang, Chem. Rev. 2008, 108, 5299–5358;
- 9bE. Malamidou-Xenikaki, S. Spyroudis, Synlett 2008, 2725–2740.
- 10M. Saito, Y. Kobayashi, S. Tsuzuki, Y. Takemoto, Angew. Chem. Int. Ed. 2017, 56, 7653–7657; Angew. Chem. 2017, 129, 7761–7765.
- 11For reviews, see:
- 11aP. M. Dewick, Medicinal Natural Products: A Biosynthetic Approach, 2nd ed., Wiley, New York, 2002;
- 11bE. Fattorusso, O. T. Scafati, Modern Alkaloids, Wiley-VCH, Weinheim, 2008.
- 12
- 12aD. Liu, G. Zhao, L. Xiang, Eur. J. Org. Chem. 2010, 3975–3984;
- 12bY.-J. Wu, Heterocyclic Scaffolds II: Topics in Heterocyclic Chemistry, Vol. 26 (Ed.: ), Springer, Heidelberg, 2010.
- 13B. Zhou, Z. Chen, Y. Yang, W. Ai, H. Tang, Y. Wu, W. Zhu, Y. Li, Angew. Chem. Int. Ed. 2015, 54, 12121–12126; Angew. Chem. 2015, 127, 12289–12294.
- 14CCDC 1813883 (3 l) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.