In Vitro Biosynthesis of the Nonproteinogenic Amino Acid Methoxyvinylglycine
Graphical Abstract
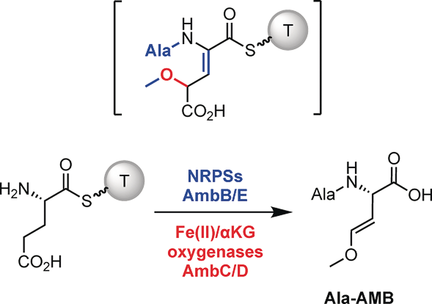
The in vitro biosynthesis of an oxyvinylglycine is reported for l-2-amino-4-methoxy-trans-3-butenoic acid (AMB). AMB is synthesized from glutamate as an alanyl-AMB dipeptide. Crafting the AMB vinyl ether requires a cascade of modifications, including a cryptic hydroxylation and a likely 2,3-dehydration.
Abstract
Oxyvinylglycines are a family of nonproteinogenic amino acids featuring an essential vinyl ether conferring mechanism-based inhibition of pyridoxal phosphate enzymes. The gene clusters for a few oxyvinylglycines are known, yet the biosynthetic origin of the vinyl ether is elusive. The in vitro biosynthesis of methoxyvinylglycine or l-2-amino-4-methoxy-trans-3-butenoic acid (AMB) is reported. It is shown that AMB is made from glutamate as an alanyl-AMB dipeptide and the rationale is provided for the N-term Ala. Using a chemical capture method, the order and timing of the modifications on non-ribosomal peptide synthetase (NRPS)-bound substrates was determined, including a cryptic hydroxylation of the Glu β-carbon. Eliminating this hydroxy group likely generates a key α,β-dehydroamino acid intermediate that facilitates decarboxylation. This work sheds light on vinyl ether biosynthesis and uncovers new NRPS chemistry.