Broadening the Scope for Fluoride-Free Synthesis of Siliceous Zeolites
Graphical Abstract
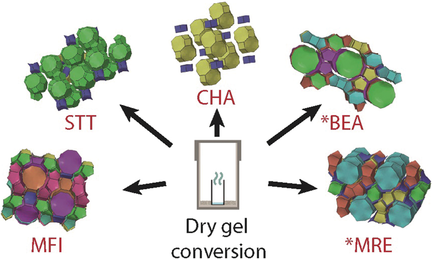
No F required: Fluoride-free synthesis of siliceous zeolites remains highly challenging—of the 234 recognized zeolite framework structures, less than 25 have been made in purely siliceous form without using fluoride. The dry gel conversion technique has been used to synthesize two new fluoride-free siliceous zeolites. Systematic studies and analysis reveal mechanistic insights into fluoride-free siliceous zeolite synthesis.
Abstract
Siliceous zeolites are ideally suited for emerging applications in gas separations, sensors, and the next generation of low-k dielectric materials, but the use of fluoride in the synthesis significantly hinders their commercialization. Herein, we show that the dry gel conversion (DGC) technique can overcome this problem. Fluoride-free synthesis of two siliceous zeolites—AMH-4 (CHA-type) and AMH-5 (STT-type), has been achieved for the first time using the method. Siliceous *BEA-, MFI-, and *MRE-type zeolites have also been synthesized to obtain insights into the crystallization process. Charge-balancing interactions between the inorganic cation, organic structure-directing agent (OSDA), and Si−O− defects are found to be an essential aspect. We quantify this factor in terms of the “OSDA charge/silica ratio” of the as-made zeolites and demonstrate that the DGC technique is broadly applicable and opens up new avenues for fluoride-free siliceous zeolite synthesis.