A Carbon Electrode Functionalized by a Tricopper Cluster Complex: Overcoming Overpotential and Production of Hydrogen Peroxide in the Oxygen Reduction Reaction
Dr. Natarajan Thiyagarajan
Institute of Chemistry, Academia Sinica, Nankang, Taipei, 11529 Taiwan) (R.O.C
Search for more papers by this authorDr. Damodar Janmanchi
Institute of Chemistry, Academia Sinica, Nankang, Taipei, 11529 Taiwan) (R.O.C
Search for more papers by this authorDr. Yi-Fang Tsai
Institute of Chemistry, Academia Sinica, Nankang, Taipei, 11529 Taiwan) (R.O.C
Search for more papers by this authorWondemagegn Hailemichael Wanna
Institute of Chemistry, Academia Sinica, Nankang, Taipei, 11529 Taiwan) (R.O.C
Search for more papers by this authorDr. Ravirala Ramu
Institute of Chemistry, Academia Sinica, Nankang, Taipei, 11529 Taiwan) (R.O.C
Search for more papers by this authorCorresponding Author
Prof. Dr. Sunney I. Chan
Institute of Chemistry, Academia Sinica, Nankang, Taipei, 11529 Taiwan) (R.O.C
Search for more papers by this authorCorresponding Author
Prof. Dr. Jyh-Myng Zen
Department of Chemistry, National Chung Hsing University, Taichung City 402, Taiwan) (R.O.C.
Search for more papers by this authorCorresponding Author
Prof. Dr. Steve S.-F. Yu
Institute of Chemistry, Academia Sinica, Nankang, Taipei, 11529 Taiwan) (R.O.C
Search for more papers by this authorDr. Natarajan Thiyagarajan
Institute of Chemistry, Academia Sinica, Nankang, Taipei, 11529 Taiwan) (R.O.C
Search for more papers by this authorDr. Damodar Janmanchi
Institute of Chemistry, Academia Sinica, Nankang, Taipei, 11529 Taiwan) (R.O.C
Search for more papers by this authorDr. Yi-Fang Tsai
Institute of Chemistry, Academia Sinica, Nankang, Taipei, 11529 Taiwan) (R.O.C
Search for more papers by this authorWondemagegn Hailemichael Wanna
Institute of Chemistry, Academia Sinica, Nankang, Taipei, 11529 Taiwan) (R.O.C
Search for more papers by this authorDr. Ravirala Ramu
Institute of Chemistry, Academia Sinica, Nankang, Taipei, 11529 Taiwan) (R.O.C
Search for more papers by this authorCorresponding Author
Prof. Dr. Sunney I. Chan
Institute of Chemistry, Academia Sinica, Nankang, Taipei, 11529 Taiwan) (R.O.C
Search for more papers by this authorCorresponding Author
Prof. Dr. Jyh-Myng Zen
Department of Chemistry, National Chung Hsing University, Taichung City 402, Taiwan) (R.O.C.
Search for more papers by this authorCorresponding Author
Prof. Dr. Steve S.-F. Yu
Institute of Chemistry, Academia Sinica, Nankang, Taipei, 11529 Taiwan) (R.O.C
Search for more papers by this authorGraphical Abstract
Abstract
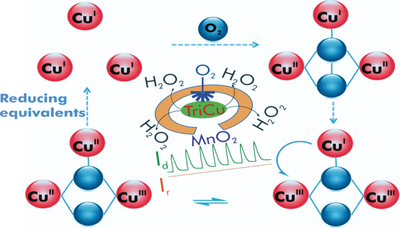
A study of the oxygen reduction reaction (ORR) on a screen printed carbon electrode surface mediated by the tricopper cluster complex Cu3(7-N-Etppz(CH2OH)) dispersed on electrochemically reduced carbon black, where 7-N-Etppz(CH2OH) is the ligand 3,3′-(6-(hydroxymethyl)-1,4-diazepane-1,4-diyl)bis(1-(4-ethyl piperazin-1-yl)propan-2-ol), is described. Onset oxygen reduction potentials of about 0.92 V and about 0.77 V are observed at pH 13 and pH 7 vs. the reversible hydrogen electrode, which are comparable to the best values reported for any synthetic copper complex. Based on half-wave potentials (E1/2), the corresponding overpotentials are about 0.42 V and about 0.68 V, respectively. Kinetic studies indicate that the trinuclear copper catalyst can accomplish the 4 e− reduction of O2 efficiently and the ORR is accompanied by the production of only small amounts of H2O2. The involvement of the copper triad in the O2 activation process is also verified.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201712226-sup-0001-misc_information.pdf799 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. Song, J. Zhang, Electrocatalytic Oxygen Reduction Reaction. PEM Fuel Cell Electrocatalysts and Catalyst Layers: Fundamentals and Applications, Springer, London, 2008.
- 2M. A. Thorseth, C. S. Letko, T. B. Rauchfuss, A. A. Gewirth, Inorg. Chem. 2011, 50, 6158–6162.
- 3
- 3aE. I. Solomon, P. Chen, M. Metz, S.-K. Lee, A. E. Palmer, Angew. Chem. Int. Ed. 2001, 40, 4570–4590;
10.1002/1521-3773(20011217)40:24<4570::AID-ANIE4570>3.0.CO;2-4 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2001, 113, 4702–4724;
- 3bE. A. Lewis, W. B. Tolman, Chem. Rev. 2004, 104, 1047–1076;
- 3cS.-K. Lee, S. D. George, W. E. Antholine, B. Hedman, K. O. Hodgson, E. I. Solomon, J. Am. Chem. Soc. 2002, 124, 6180–6193.
- 4N. Mano, V. Soukharev, A. Heller, J. Phys. Chem. B 2006, 110, 11180–11187.
- 5Y. C. Weng, F.-R. F. Fan, A. J. Bard, J. Am. Chem. Soc. 2005, 127, 17576–17577.
- 6
- 6aA. J. Augustine, C. Kjaergaard, M. Qayyum, L. Ziegler, D. J. Kosman, K. O. Hodgson, B. Hedman, E. I. Solomon, J. Am. Chem. Soc. 2010, 132, 6057–6067;
- 6bM. A. Thorseth, C. E. Tornow, E. C. M. Tse, A. A. Gewirth, Coord. Chem. Rev. 2013, 257, 130–139;
- 6cY.-T. Xi, P.-J. Wei, R.-C. Wang, J.-G. Liu, Chem. Commun. 2015, 51, 7455–7458;
- 6dF. He, L. Mi, F. Shen, X. Chen, Y. Yang, H. Mei, S. Liu, T. Mori, Y. Zhang, J. Mater. Chem. A 2017, 5, 17413–17420.
- 7
- 7aC. C. L. McCrory, X. Ottenwaelder, T. D. P. Stack, C. E. D. Chidsey, J. Phys. Chem. A 2007, 111, 12641–12650;
- 7bC. C. L. McCrory, A. Devadoss, X. Ottenwaelder, R. D. Lowe, T. D. P. Stack, C. E. D. Chidsey, J. Am. Chem. Soc. 2011, 133, 3696–3699.
- 8
- 8aL. Tahsini, H. Kotani, Y.-M. Lee, J. Cho, W. Nam, K. D. Karlin, S. Fukuzumi, Chem. Eur. J. 2012, 18, 1084–1093;
- 8bA. Gomila, N. L. Poul, J.-M. Kerbaol, N. Cosquer, S. Triki, B. Douziech, F. Conan, Y. L. Mest, Dalton Trans. 2013, 42, 2238–2253;
- 8cM. A. Thorseth, C. S. Letko, E. C. M. Tse, T. B. Rauchfuss, A. A. Gewirth, Inorg. Chem. 2013, 52, 628–634.
- 9M. S. Thorum, J. Yadav, A. A. Gewirth, Angew. Chem. Int. Ed. 2009, 48, 165–167; Angew. Chem. 2009, 121, 171–173.
- 10E. C. M. Tse, D. Schilter, D. L. Gray, T. B. Rauchfuss, A. A. Gewirth, Inorg. Chem. 2014, 53, 8505–8516.
- 11
- 11aA. P. Cole, D. E. Root, P. Mukherjee, E. I. Solomon, T. D. P. Stack, Science 1996, 273, 1848–1850;
- 11bM. Casarin, C. Corvaja, C. D. Nicola, D. Falcomer, L. Franco, M. Monari, L. Pandolfo, C. Pettinari, F. Piccinelli, Inorg. Chem. 2005, 44, 6265–6276;
- 11cW. Ouellette, M. H. Yu, C. J. O. Connor, D. Hagrman, J. Zubieta, Angew. Chem. Int. Ed. 2006, 45, 3497–3500; Angew. Chem. 2006, 118, 3577–3580;
- 11dD. Maiti, J. S. Woertink, R. A. Ghiladi, E. I. Solomon, K. D. Karlin, Inorg. Chem. 2009, 48, 8342–8356;
- 11eE. Y. Tsui, M. W. Day, T. Agapie, Angew. Chem. Int. Ed. 2011, 50, 1668–1672; Angew. Chem. 2011, 123, 1706–1710.
- 12
- 12aP. P.-Y. Chen, R. B.-G. Yang, J. C.-M. Lee, S. I. Chan, Proc. Natl. Acad. Sci. USA 2007, 104, 14570–14574;
- 12bS. I. Chan, C. Y.-C. Chien, C. S.-C. Yu, P. Nagababu, S. Maji, P. P.-Y. Chen, J. Catal. 2012, 293, 186–194;
- 12cS. Maji, J. C.-M. Lee, Y.-J. Lu, C.-L. Chen, M.-C. Hung, P. P.-Y. Chen, S. S.-F. Yu, S. I. Chan, Chem. Eur. J. 2012, 18, 3955–3968.
- 13
- 13aS. I. Chan, Y.-J. Lu, P. Nagababu, S. Maji, M.-C. Hung, M. M. Lee, I.-J. Hsu, P. D. Minh, J. C.-H. Lai, K. Y. Ng, S. Ramalingam, S. S.-F. Yu, M. K. Chan, Angew. Chem. Int. Ed. 2013, 52, 3731–3735; Angew. Chem. 2013, 125, 3819–3823;
- 13bP. P.-Y. Chen, P. Nagababu, S. S.-F. Yu, S. I. Chan, ChemCatChem 2014, 6, 429–437;
- 13cC.-C. Liu, C.-Y. Mou, S. S.-F. Yu, S. I. Chan, Energy Environ. Sci. 2016, 9, 1361–1374.
- 14
- 14aS. I. Chan, K. H.-C. Chen, S. S.-F. Yu, C.-L. Chen, S. S.-J. Kuo, Biochemistry 2004, 43, 4421–4430;
- 14bS. I. Chan, S. S.-F. Yu, Acc. Chem. Res. 2008, 41, 969–979.
- 15S. Pintado, S. Goberna-Ferron, E. C. Escudero-Adan, J. R. Galan-Mascaros, J. Am. Chem. Soc. 2013, 135, 13270–13273.
- 16E. Laviron, J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28.
- 17M. L. Pegis, B. A. McKeown, N. Kumar, K. Lang, D. J. Wasylenko, X. P. Zhang, S. Raugei, J. M. Mayer, ACS Cent. Sci. 2016, 2, 850–856.
- 18M. Gong, Y. Li, H. Wang, Y. Liang, J. Z. Wu, J. Zhou, J. Wang, T. Regier, F. Wei, H. Dai, J. Am. Chem. Soc. 2013, 135, 8452–8455.
- 19T.-H. Yang, C.-Y. Liao, J.-L. Chang, C.-H. Lien, J.-M. Zen, Electroanalysis 2009, 21, 2390–2394.
- 20
- 20aX. Ge, A. Sumboja, D. Wuu, T. An, B. Li, F. W. T. Goh, T. S. A. Hor, Y. Zong, Z. Liu, ACS Catal. 2015, 5, 4643–4667;
- 20bN. Ramaswamy, S. Mukerjee, Adv. Phys. Chem. 2012, DOI 10.1155/2012/491604.
- 21F. H. B. Lima, J. Zhang, M. H. Shao, K. Sasaki, M. B. Vukmirovic, E. A. Ticianelli, R. R. Adzic, J. Phys. Chem. C 2007, 111, 404–410.
- 22J. J. Zuckerman, A. P. Hagen, Inorganic Reactions and Methods, The Formation of Bonds to N, P, As, Sb, Bi, Wiley, New York, 2009.
- 23E. C. M. Tse, C. J. Barile, N. A. Kirchschlager, Y. Li, J. P. Gewargis, S. C. Zimmerman, A. Hosseini, A. A. Gewirth, Nat. Mater. 2016, 15, 754–759.